中国海草块茎中新的多羟基葫芦烷型三萜及其细胞毒活性
IF 2.6
3区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
对中国海草块茎95%乙醇提取物进行植物化学研究,分离出五种以前未描述的葫芦烷型三萜(1-5,命名为hemslencins G-K),以及五种已知的类似物(6-10)。通过综合光谱分析(UV、IR、HR-ESI-MS、1D和2D NMR数据)对这些化合物的结构进行了鉴定。化合物1、2、7、9和10为24氧合葫芦烷型三萜,在海姆斯利亚属中很少发现。测定了化合物1 ~ 10对MCF-7、HCT-116、HeLa和HepG2癌细胞的细胞毒活性,化合物1和化合物8 ~ 10对MCF-7、HCT-116、HeLa和HepG2的细胞毒活性,结果表明化合物1和化合物8 ~ 10的细胞毒活性为0.382 ~ 13.77 μM。进一步的机制研究表明,化合物1剂量依赖性地诱导MCF-7细胞凋亡并导致G0/G1期细胞周期阻滞。本文章由计算机程序翻译,如有差异,请以英文原文为准。
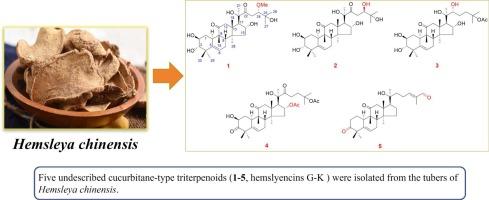
New polyhydroxy cucurbitane-type triterpenoids from the tubers of Hemsleya chinensis and their cytotoxic activities
Phytochemical investigation of the 95 % ethanol extract of the tubers of Hemsleya chinensis led to the isolation of five previously undescribed cucurbitane-type triterpenoids (1–5, named hemslyencins G-K), along with five known analogues (6–10). The structures of these compounds were elucidated through comprehensive spectroscopic analysis (UV, IR, HR-ESI-MS, 1D and 2D NMR data). Compounds 1, 2, 7, 9, and 10 are characterized as 24‑oxygenated cucurbitane-type triterpenoids, were rarely found in the genus Hemsleya. The cytotoxic activities of 1–10 were evaluated against MCF-7, HCT-116, HeLa, and HepG2 cancer cell lines, in which compounds 1 and 8–10 exhibited significant cytotoxic activities with the value of 0.382–13.77 μM. Further mechanism studies displayed that compound 1 dose-dependently induced apoptosis and caused G0/G1 phase cell cycle arrest in MCF-7 cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fitoterapia
医学-药学
CiteScore
5.80
自引率
2.90%
发文量
198
审稿时长
1.5 months
期刊介绍:
Fitoterapia is a Journal dedicated to medicinal plants and to bioactive natural products of plant origin. It publishes original contributions in seven major areas:
1. Characterization of active ingredients of medicinal plants
2. Development of standardization method for bioactive plant extracts and natural products
3. Identification of bioactivity in plant extracts
4. Identification of targets and mechanism of activity of plant extracts
5. Production and genomic characterization of medicinal plants biomass
6. Chemistry and biochemistry of bioactive natural products of plant origin
7. Critical reviews of the historical, clinical and legal status of medicinal plants, and accounts on topical issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


