混合碱性/碱土金属硼磷酸盐的合成、结构表征及非线性光学性质
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
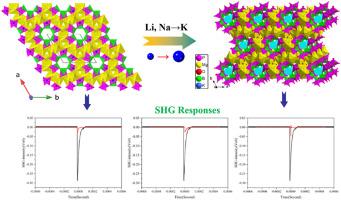
硼磷酸盐由于其独特的结构特征和优异的性能而引起了人们的广泛关注。一价碱金属和二价碱土金属加入硼磷酸盐体系后,晶体结构明显多样化。采用低温水热法合成了一种新型硼磷酸盐KMg [B(PO4)2](H2O)2和两种非线性光学材料NaMg(H2O)2(BP2O8) (H2O)和LiMg(H2O)2(BP2O8) (H2O)。这些化合物在P6122的同一极性空间群中结晶。KMg [B(PO4)2](H2O)2、NaMg(H2O)2(BP2O8) (H2O)和LiMg(H2O)2(BP2O8) (H2O)的基本结构单元由BO4四面体、PO4四面体和MgO6八面体组成。在这些结构中,两个BO4四面体和两个PO4四面体形成螺旋链,而MgO6八面体通过共享氧原子与四个PO4四面体连接,连接相邻的螺旋链形成三维(3D)框架。碱金属(K, Na, Li)位于这些框架的空隙中。KMg[B(PO4)2](H2O)2、NaMg(H2O)2(BP2O8) (H2O)和LiMg(H2O)2(BP2O8) (H2O)在紫外区表现出中等的二次谐波产生(SHG)响应,分别为~ 0.13 × KDP、~ 0.10 × KDP和~ 0.17 × KDP。讨论了合成方法、拓扑结构、元素分析、热稳定性、红外光谱、拉曼光谱、UV-Vis漫反射光谱和NLO性能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Syntheses, structural characterization, and nonlinear optical properties of mixed alkaline/alkaline earth metal borophosphates
Borophosphates have attracted significant attention due to their unique structural characteristics and exceptional properties. The incorporation of monovalent alkaline metals and divalent alkaline earth metals into the borophosphate system has significantly diversified the crystal structures. A new borophosphate, KMg [B(PO4)2](H2O)2, along with two previously reported nonlinear optical (NLO) materials, NaMg(H2O)2(BP2O8) (H2O) and LiMg(H2O)2(BP2O8) (H2O), were synthesized using low-temperature hydrothermal methods. These compounds crystallized in the same polar space group of P6122. The fundamental building units of KMg [B(PO4)2](H2O)2, NaMg(H2O)2(BP2O8) (H2O) and LiMg(H2O)2(BP2O8) (H2O) consist of BO4 tetrahedra, PO4 tetrahedra and MgO6 octahedra. In these structures, two BO4 tetrahedra and two PO4 tetrahedra form helical chains, while the MgO6 octahedra connect to four PO4 tetrahedra through shared oxygen atoms, linking adjacent helical chains to form a three-dimensional (3D) framework. Alkaline metals (K, Na, Li) are situated within the voids of these frameworks. KMg[B(PO4)2](H2O)2, NaMg(H2O)2(BP2O8) (H2O) and LiMg(H2O)2(BP2O8) (H2O) exhibit moderate second harmonic generation (SHG) responses in the ultraviolet region, with of ∼0.13 × KDP, ∼0.10 × KDP and ∼0.17 × KDP, respectively. The syntheses and topological structures, elemental analyses, thermal stability, infrared spectroscopy, Raman spectroscopy, UV–Vis diffuse reflectance spectroscopy and NLO properties are discussed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


