利用抗tnfr1治疗对肥胖雄性小鼠中风后的炎症和运动功能的影响有限
IF 2.6
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
摘要
肥胖是一个重要的全球健康问题,它通过慢性低度全身炎症和代谢失调加剧缺血性中风的风险和严重程度。肿瘤坏死因子(TNF)信号,特别是通过其TNF受体1 (TNFR1),参与肥胖驱动的炎症和不良卒中结局。为了评估TNFR1阻断作为一种治疗策略的潜力,我们采用了饮食诱导的缺血性卒中肥胖模型。雄性C57BL/6小鼠在光血栓诱导脑卒中前6周分别饲喂常规饮食或高脂饮食,并用tnfr1中和抗体或同型对照抗体治疗。结果通过运动功能评估、全身炎症生物标志物测量和脑组织分析来评估,包括小胶质细胞、星形胶质细胞、少突胶质细胞、髓磷脂完整性和卒中大小的评估。结果显示,与非肥胖对照组相比,肥胖小鼠在中风后表现出更严重的运动缺陷和更高的全身性炎症。用抗tnfr1抗体治疗并没有改善肥胖小鼠中风后增加的功能缺陷,但确实提高了白细胞介素-6细胞因子水平,而对非肥胖对照组没有影响。此外,抗tnfr1治疗不影响梗死面积,小胶质细胞和星形胶质细胞的反应性,或髓磷脂完整性在两个饮食组。总的来说,tnfr1靶向治疗对肥胖加重的卒中结果影响有限,这表明需要探索更广泛或联合免疫调节的方法。这些发现还强调了考虑卒中中肥胖等合并症的重要性,因为它们会影响疾病进展和治疗效果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
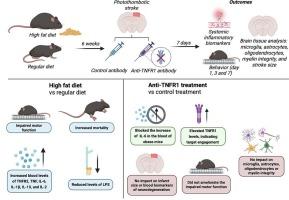
Harnessing anti-TNFR1 therapy has a limited impact on inflammation and motor function after stroke in obese male mice
Obesity is a significant global health concern that exacerbates the risk and severity of ischemic stroke through chronic low-grade systemic inflammation and metabolic dysregulation. Tumor necrosis factor (TNF) signaling, particularly through its TNF receptor 1 (TNFR1), is involved in obesity-driven inflammation and adverse stroke outcomes. To assess the potential of TNFR1 blockade as a treatment strategy, we employed a diet-induced obesity model of ischemic stroke. Male C57BL/6 mice were fed a regular diet or high-fat diet for 6 weeks prior to stroke induction via photothrombosis and treated with either a TNFR1-neutralizing antibody or isotype control antibody. Outcomes were evaluated using motor function assessments, systemic inflammatory biomarker measurements, and brain tissue analysis, including evaluation of microglia, astrocytes, oligodendrocytes, myelin integrity, and stroke size. Results showed that obese mice exhibited worsened motor deficits and heightened systemic inflammation following stroke compared to non-obese controls. Treatment with anti-TNFR1 antibody did not ameliorate the increased functional deficits but did improve interleukin-6 cytokines levels after stroke in obese mice, while it had no effects on non-obese controls. Moreover, anti-TNFR1 therapy did not impact infarct size, microglial and astrocyte reactivity, or myelin integrity in both dietary groups. Overall, TNFR1-targeted therapy had a limited impact on obesity-exacerbated stroke outcomes, suggesting the need to explore broader or combination immunomodulatory approaches. These findings also emphasize the importance of considering comorbidities such as obesity in stroke, as they can influence both disease progression and treatment effectiveness.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


