FOXP基因调控浦肯野细胞多样性和小脑形态发生。
IF 20
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
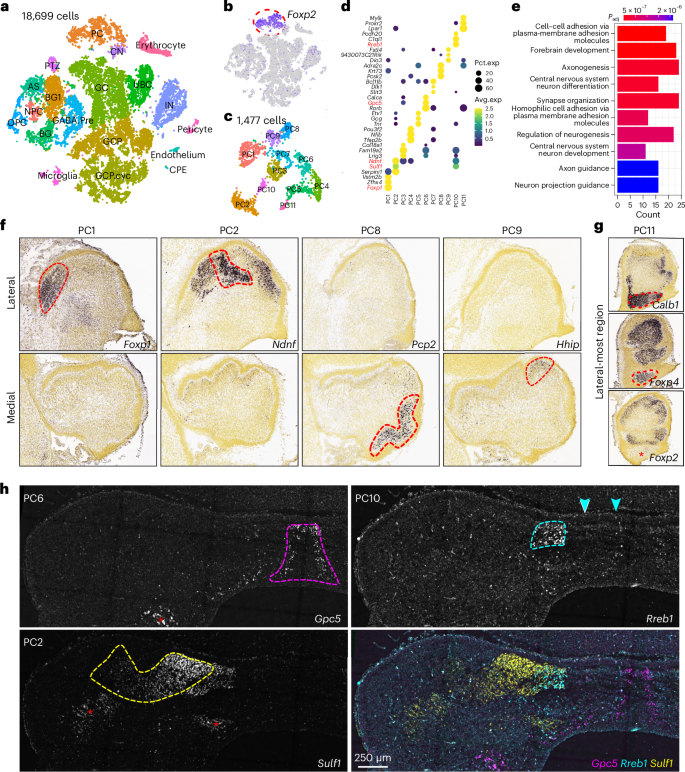
小脑浦肯野细胞(PCs)是小脑皮层唯一的输出神经元,对运动协调、学习和电路形成至关重要。虽然功能多样,但PC异质性的程度和这种多样性的分子驱动因素仍不清楚。利用单细胞RNA测序,我们在胚胎小鼠小脑中鉴定出至少11种分子上不同的PC亚型。空间重建显示,这些亚型以胚胎模式组织,预测成人小脑结构的关键特征,包括纵向条纹和小叶特异性。pc亚型身份由Foxp1、Foxp2和Foxp4的组合表达来定义。Foxp1和Foxp2的基因缺失会破坏PC多样化和小脑模式,包括Foxp1+亚型的缺失和小脑半球形成的失败。Foxp1+ PCs在胎儿人类小脑中富集,但在小鸡中很少见,这表明Foxp1+ PCs在小脑进化中起作用。这些发现揭示了早期PC多样化,并确定Foxp1+ PC是小脑半球发育的关键调节因子。本文章由计算机程序翻译,如有差异,请以英文原文为准。

FOXP genes regulate Purkinje cell diversity and cerebellar morphogenesis
Cerebellar Purkinje cells (PCs), the sole output neurons of the cerebellar cortex, are essential for motor coordination, learning and circuit formation. While functionally diverse, the extent of PC heterogeneity and the molecular drivers of this diversity remain unclear. Using single-cell RNA sequencing, we identified at least 11 molecularly distinct PC subtypes in the embryonic mouse cerebellum. Spatial reconstruction revealed that these subtypes are organized in embryonic patterns that predict key features of adult cerebellar architecture, including longitudinal stripes and lobular specificities. PC-subtype identity is defined by the combinatorial expression of Foxp1, Foxp2 and Foxp4. Genetic deletion of Foxp1 and Foxp2 disrupts PC diversification and cerebellar patterning, including the loss of a Foxp1+ subtype and the failure of cerebellar hemisphere formation. Foxp1+ PCs are enriched in the fetal human cerebellum but are rare in chick, suggesting a role in cerebellar evolution. These findings uncover early PC diversification and identify Foxp1+ PCs as critical regulators of cerebellar hemispheric development. The Li lab mapped molecularly distinct Purkinje cell (PC) subtypes in 3D and linked them to adult cerebellar architecture. They found that Foxp1/Foxp2 are essential for PC diversity and that Foxp1+ PCs are required for the formation of the cerebellar hemisphere.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


