高压下对全氟甲苯过氧化酸存在下全氟丙基乙烯醚自由基聚合动力学
IF 0.6
4区 化学
Q4 CHEMISTRY, APPLIED
引用次数: 0
摘要
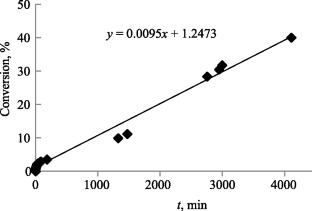
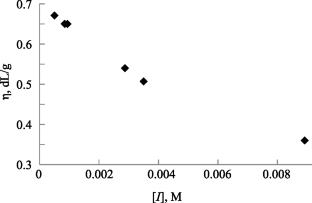
研究了在自由基引发剂存在下,全氟丙基乙烯醚(PFPE)在压力为264 ~ 1056 MPa、温度为323 ~ 368 K下的自由基聚合动力学。在不同压力下测定活化能:P = 10.56 kbar时,Eact = 135.4 kJ/mol (32.4 kcal/mol); P =7.04 kbar时,Eact = 108.3 kJ/mol (25.9 kcal/mol)。在T = 353 K时测定了总聚合速率ΔV0≠= -14.6 cm3/mol的活化体积。计算了不同压力下材料起始活化能[P = 10.56 kbar, Ei = 123.3 kJ/mol (29.5 kcal/mol), P = 7.04 kbar, Ei = 88.3 kJ/mol (21.1 kcal/mol)]。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Kinetics of Radical Polymerization of Perfluoropropylvinyl Ether in the Presence of p-Perfluorotoluylic Acid Peroxide at High Pressures
The kinetics of radical polymerization of perfluoropropylvinyl ether (PFPE) was investigated in the presence of a radical initiator at pressures of 264–1056 MPa and temperatures of 323–368 K. Activation energies were determined at different pressures: at P = 10.56 kbar Eact = 135.4 kJ/mol (32.4 kcal/mol), at P =7.04 kbar Eact = 108.3 kJ/mol (25.9 kcal/mol). The activation volume of the total polymerization rate ΔV0≠ = –14.6 cm3/mol was determined at T = 353 K. The activation energies of the material initiation were calculated at different pressures [at P = 10.56 kbar, Ei = 123.3 kJ/mol (29.5 kcal/mol), at P = 7.04 kbar, Ei = 88.3 kJ/mol (21.1 kcal/mol)].
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.60
自引率
11.10%
发文量
63
审稿时长
2-4 weeks
期刊介绍:
Russian Journal of Applied Chemistry (Zhurnal prikladnoi khimii) was founded in 1928. It covers all application problems of modern chemistry, including the structure of inorganic and organic compounds, kinetics and mechanisms of chemical reactions, problems of chemical processes and apparatus, borderline problems of chemistry, and applied research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


