二氯单硫代氨基脲锌(ii)†的简单机械化学制备
IF 2.5
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
采用快速、简便、重现性好的机械化学方法制备了分子式为ZnCl2[CH5N3S]的二氯单硫代氨基脲锌(II)。第一排过渡金属与硫代氨基脲、硫代氨基脲或其衍生物的配位化合物因其抗菌和抗真菌的特性而引起了人们的兴趣。因此,将更环保的合成方法带到生产这些有用化合物的桌子上是可取的。采用x射线粉末衍射、质子核磁谱、红外光谱、熔点和磁化率等方法对产物ZnCl2[CH5N3S]进行了表征。本文章由计算机程序翻译,如有差异,请以英文原文为准。
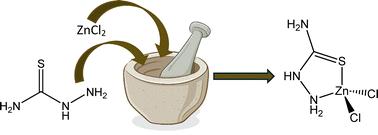
Simple mechanochemical preparation of dichloromonothiosemicarbazidezinc(ii)†
Dichloromonothiosemicarbazidezinc(II), with the molecular formula ZnCl2[CH5N3S], has been prepared via a fast, easy, and reproducible mechanochemical method. Coordination compounds using first-row transition metals with ligands of thiosemicarbazide, thiosemicarbazone, or their derivatives have been of recent interest for their antibacterial and antifungal properties. Thus, bringing greener synthesis methods to the table for production of such useful compounds is desirable. The pure product, ZnCl2[CH5N3S], was characterized using X-ray powder diffraction, proton nuclear magnetic spectroscopy, infra-red spectroscopy, melting point, and magnetic susceptibility measurements.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

New Journal of Chemistry
化学-化学综合
CiteScore
5.30
自引率
6.10%
发文量
1832
审稿时长
2 months
期刊介绍:
A journal for new directions in chemistry
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


