小麦近等基因系的比较防御分析揭示了小麦抗条锈病基因的机制多样性,使小麦抗条锈病基因具有统一的侵染类型
IF 3.3
3区 农林科学
Q2 PLANT SCIENCES
引用次数: 0
摘要
小麦(Triticum aestivum L.)是一种主要的粮食作物,由于引起条锈病的小麦锈病(Pst),小麦生产面临重大挑战。寄主植物抗性作为一种有效的病害管理策略,需要更深入地了解抗条锈病基因所赋予的抗性机制。在本研究中,携带不同Yr基因(Yr5、Yr10、Yr15、Yr24和YrSP)的近等基因系(NILs)在接种新出现的Pst致病型238S119后表现出统一的感染类型(IT ' 0 '),但表现出不同的防御机制。吸器发育期间(72 ~ 120 hpi)抗性出现分化,孢子萌发和附着胞形成无差异。在Avocet + Yr5、Avocet + Yr10和Avocet + Yr24中,早期木质素化和过敏反应(hypersensitive response, HR)更为明显,而延迟胼质沉积则是基因特异性的,在Avocet + Yr5和Avocet + Yr15中积累更强,这突出了抵御病原体入侵的结构强化的差异。生化分析揭示了Yr基因特异性的贡献。牛油果+ Yr10的苯丙氨酸解氨酶(PAL)活性、牛油果+ Yr5的酪氨酸解氨酶(TAL)活性、牛油果+ Yr15的多酚氧化酶(PPO)活性和牛油果+ Yr24的过氧化氢酶(CAT)活性均被高度诱导。此外,多变量分析表明,较高的PAL、TAL、PPO、CAT和酚类物质与抗性相关,而较高的过氧化氢(H2O2)和丙二醛(MDA)水平与敏感性相关。这些发现表明,苯丙素代谢在连接苯酚与木质素生物合成和有效的氧化应激管理中具有关键作用。尽管有类似的外部抗性,但这些Yr基因激活不同的结构和生化反应来阻止Pst感染。了解这种多样性对于在小麦育种中设计有效、持久的抗性策略至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
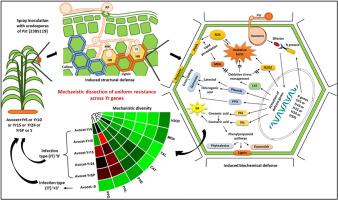
Comparative defense profiling in wheat near-isogenic lines reveals mechanistic diversity among stripe rust resistance (Yr) genes conferring uniform infection type against Puccinia striiformis f. sp. tritici
Wheat (Triticum aestivum L.), a chief staple food crop, faces significant production challenges due to Puccinia striiformis f. sp. tritici (Pst), responsible for stripe rust. Host plant resistance, being an effective disease management strategy, necessitates a deeper understanding of resistance mechanisms conferred by stripe rust-resistance (Yr) genes. In this study, near-isogenic lines (NILs) carrying different Yr genes (Yr5, Yr10, Yr15, Yr24, and YrSP) displayed a uniform infection type (IT‘0’) upon inoculation with the emerging virulent Pst pathotype 238S119, yet exhibited divergent defense mechanisms. While spore germination and appressorium formation showed no differences, resistance divergence emerged during haustorial development (72–120 hpi). Early lignification and hypersensitive response (HR) were more pronounced in Avocet + Yr5, Avocet + Yr10, and Avocet + Yr24, while delayed callose deposition was gene-specific, with stronger accumulation in Avocet + Yr5 and Avocet + Yr15, highlighting variation in structural reinforcement against pathogen ingress. Biochemical profiling revealed Yr gene-specific contributions. Phenylalanine ammonia lyase (PAL) activity was highly induced in Avocet + Yr10, tyrosine ammonia lyase (TAL) in Avocet + Yr5, polyphenol oxidase (PPO) in Avocet + Yr15, and catalase (CAT) in Avocet + Yr24. Further, multivariate analysis showed that higher PAL, TAL, PPO, CAT, and phenolics were associated with resistance, while higher hydrogen peroxide (H2O2) and malondialdehyde (MDA) levels correlated with susceptibility. These findings suggest the crucial role of phenylpropanoid metabolism in linking phenol to lignin biosynthesis and effective oxidative stress management in disease resistance. Despite similar external resistance, these Yr genes activate different structural and biochemical responses to block Pst infection. Understanding such diversity is critical for designing effective, durable resistance strategies in wheat breeding.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
4.30
自引率
7.40%
发文量
130
审稿时长
38 days
期刊介绍:
Physiological and Molecular Plant Pathology provides an International forum for original research papers, reviews, and commentaries on all aspects of the molecular biology, biochemistry, physiology, histology and cytology, genetics and evolution of plant-microbe interactions.
Papers on all kinds of infective pathogen, including viruses, prokaryotes, fungi, and nematodes, as well as mutualistic organisms such as Rhizobium and mycorrhyzal fungi, are acceptable as long as they have a bearing on the interaction between pathogen and plant.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


