高强度高强度增强盐还原鱼糜凝胶特性的研究:内源性谷氨酰胺转肽类酶激活和蛋白酶抑制的双重调控
IF 9.7
1区 化学
Q1 ACOUSTICS
引用次数: 0
摘要
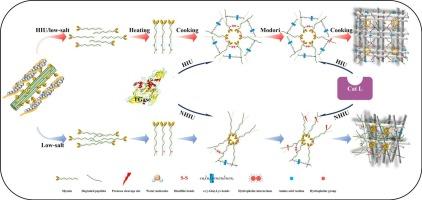
先前的研究表明,高强度超声(HIU)可以提高1.5% NaCl盐还原鱼糜凝胶的凝胶性能。了解其机制,特别是关键内源性酶的作用,对于hiu辅助调节盐还原鱼糜凝胶具有令人满意的结构性质至关重要。本研究采用酶特异性添加剂,从刺穿性能、蛋白质降解和交联、动态流变性能、水分分布和微观结构等方面考察了HIU对盐还原(1.5% NaCl)鱼糜凝胶化性能的增强作用。结果表明,CaCl2和E-64显著提高了鱼糜凝胶的破断力。这种改善是因为Ca2+激活了内源性谷氨酰胺转胺酶(eTGase),非二硫共价键的形成增加,而E-64通过抑制半胱氨酸蛋白酶减少了蛋白质降解。HIU和CaCl2的结合降低了加热过程中的凝固温度,促进了非二硫共价键的形成,形成了三维凝胶网络,有效地捕获了更多的水。因此,穿刺性能得到了改善。HIU和E-64的结合有效地抑制了蛋白质的降解,在霉菌期结束时,tca可溶性肽减少,G′增加。值得注意的是,HIU与NH4Cl的结合虽然改善了凝胶性能,但仍未能形成典型的三维凝胶网络。综上所示,HIU通过激活eTGase和抑制半胱氨酸蛋白酶的双重机制增强了鱼糜的凝胶特性,其中eTGase的激活作用大于蛋白酶的抑制作用,为HIU辅助生产盐还原鱼糜凝胶提供了理论基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Insights into HIU enhancement of salt-reduced surimi gelation properties: Dual regulation of endogenous transglutaminase activation and protease inhibition
Previous studies have demonstrated that high-intensity ultrasound (HIU) could enhance the gel properties of salt-reduced (1.5 % NaCl) surimi gels. Understanding its mechanism, particularly the role of key endogenous enzymes, is essential for HIU-assisted modulation of salt-reduced surimi gels with satisfactory textural properties. In this study, enzyme-specific additives were employed to investigate the enhancement effect of HIU on the gelation properties of salt-reduced (1.5 % NaCl) surimi in terms of puncture properties, protein degradation and crosslinking, dynamic rheological properties, water distribution and microstructures. Results showed that CaCl2 and E-64 significantly increased the breaking force of surimi gels. This improvement occurred because Ca2+ activated endogenous transglutaminase (eTGase), as evidenced by the increased formation of non-disulfide covalent bonds, while E-64 reduced protein degradation by inhibiting cysteine protease. The combination of HIU and CaCl2 lowered the setting temperature during heating and facilitated the formation of non-disulfide covalent bonds, contributing to a three-dimensional gel network that effectively trapped more water. Consequently, the puncture properties were improved. The combination of HIU and E-64 effectively inhibited protein degradation, as evidenced by decreased TCA-soluble peptides and increased G′ at the end of the modori stage. Notably, although the combination of HIU and NH4Cl improved the gel properties, it still failed to form the typical three-dimensional gel network. Collectively, HIU enhanced the gelation properties of surimi through dual mechanisms of eTGase activation and cysteine protease inhibition, with eTGase activation contributing more substantially than protease inhibition, providing a theoretical foundation for HIU-assisted production of salt-reduced surimi gels.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Ultrasonics Sonochemistry
化学-化学综合
CiteScore
15.80
自引率
11.90%
发文量
361
审稿时长
59 days
期刊介绍:
Ultrasonics Sonochemistry stands as a premier international journal dedicated to the publication of high-quality research articles primarily focusing on chemical reactions and reactors induced by ultrasonic waves, known as sonochemistry. Beyond chemical reactions, the journal also welcomes contributions related to cavitation-induced events and processing, including sonoluminescence, and the transformation of materials on chemical, physical, and biological levels.
Since its inception in 1994, Ultrasonics Sonochemistry has consistently maintained a top ranking in the "Acoustics" category, reflecting its esteemed reputation in the field. The journal publishes exceptional papers covering various areas of ultrasonics and sonochemistry. Its contributions are highly regarded by both academia and industry stakeholders, demonstrating its relevance and impact in advancing research and innovation.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


