用Cyanex272在树脂上固定钨(VI)和钼(VI)的选择性分配
IF 4.6
2区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
International Journal of Refractory Metals & Hard Materials
Pub Date : 2025-08-05
DOI:10.1016/j.ijrmhm.2025.107362
引用次数: 0
摘要
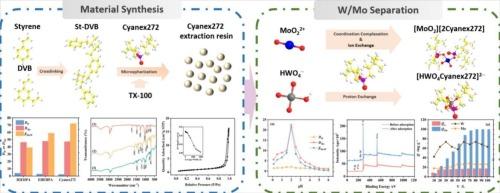
钨酸铵溶液中钨(W)和钼(Mo)的分离是湿法冶金中的一个重大挑战,因为它们的化学性质几乎相同。本研究通过萃取色谱法合成了一种新型的cyanex272 -固定化苯乙烯-二乙烯基苯(St-DVB)树脂,实现了W和Mo的选择性分配。在静态和动态条件下的系统研究表明,pH 2.0是最大限度地吸附钼的最佳条件。热力学分析揭示了一个熵驱动的吸热吸附机制,其中升高的温度增强了钼的选择性,同时保持了良好的动力学。FTIR和XPS分析表明MoO22+与Cyanex272中的磷酸基团(-PO (O−))具有配位性。吸附等温线与Langmuir模型一致,表明单层化学吸附以配位为主。动力学分析显示了伪二阶行为,外部传质和颗粒内扩散是连续的限速步骤。氨溶液(2.0 mol·L−1)解吸效率达到99.9%,实现了树脂再生和闭环资源回收。柱吸附表现出优异的性能,从工业级饲料(100 g·L−1 W, 0.5 g·L−1 Mo)中去除99.88%的Mo,同时将出水Mo保持在0.6 mg·L−1以下,分离系数(βMo/W)为9.63 × 105。纯化后的溶液符合高纯度仲钨酸铵(APT)生产的严格标准。通过溶剂萃取选择性与离子交换实用性的协同作用,该方法为持续存在的WMo分离挑战提供了一种工业上可扩展且环境可持续的解决方案,在先进材料制造中具有潜在的应用前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Selective partitioning of tungsten (VI) and molybdenum (VI) through the immobilization of Cyanex272 on resin
The separation of tungsten (W) and molybdenum (Mo) from ammonium tungstate solutions poses a significant challenge in hydrometallurgy due to their nearly identical chemical properties. This study synthesized a novel Cyanex272-immobilized styrene-divinylbenzene (St-DVB) resin to achieve selective partitioning of W and Mo through an extractive chromatography approach. Systematic investigations under static and dynamic conditions identify pH 2.0 as optimal for maximizing Mo adsorption. Thermodynamic analysis revealed an entropy-driven endothermic adsorption mechanism, where elevated temperature enhances Mo selectivity while maintaining favorable kinetics. FTIR, XPS analysis demonstrated the coordination of MoO22+ with the phosphinic acid groups (–PO(O−)) in Cyanex272. Adsorption isotherms aligned with the Langmuir model, indicating that monolayer chemisorption is dominated by ligand-metal coordination. Kinetic analysis revealed pseudo-second-order behavior, with external mass transfer and intraparticle diffusion as sequential rate-limiting steps. Ammonia solution (2.0 mol·L−1) achieved >99.9 % desorption efficiency, enabling resin regeneration and closed-loop resource recovery. Column adsorption demonstrated exceptional performance, removing 99.88 % Mo from industrial-grade feed (100 g·L−1 W, 0.5 g·L−1 Mo) while maintaining effluent Mo below 0.6 mg·L−1, with a separation factor (βMo/W) of 9.63 × 105. The purified solution met stringent standards for high-purity ammonium paratungstate (APT) production. By synergizing solvent extraction selectivity with ion-exchange practicality, this method offers an industrially scalable and environmentally sustainable solution to the persistent W![]() Mo separation challenge, with potential applications in advanced material manufacturing.
Mo separation challenge, with potential applications in advanced material manufacturing.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.00
自引率
13.90%
发文量
236
审稿时长
35 days
期刊介绍:
The International Journal of Refractory Metals and Hard Materials (IJRMHM) publishes original research articles concerned with all aspects of refractory metals and hard materials. Refractory metals are defined as metals with melting points higher than 1800 °C. These are tungsten, molybdenum, chromium, tantalum, niobium, hafnium, and rhenium, as well as many compounds and alloys based thereupon. Hard materials that are included in the scope of this journal are defined as materials with hardness values higher than 1000 kg/mm2, primarily intended for applications as manufacturing tools or wear resistant components in mechanical systems. Thus they encompass carbides, nitrides and borides of metals, and related compounds. A special focus of this journal is put on the family of hardmetals, which is also known as cemented tungsten carbide, and cermets which are based on titanium carbide and carbonitrides with or without a metal binder. Ceramics and superhard materials including diamond and cubic boron nitride may also be accepted provided the subject material is presented as hard materials as defined above.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


