脱质子效应使Fe-N4-C催化剂氧还原电催化活性位密度加倍
IF 8.9
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
具有Fe-N4结构的铁-氮-碳(Fe-N-C)材料被认为是稀有和贵重铂(Pt)在氧还原反应中最有前途的替代品。特别是含n胺聚合物、铁盐和碳载体的前驱体混合物的高温热解,已成为合成高性能Fe- n -c催化剂的常用方法。胺单体的氧化聚合通常可以在酸性条件下进行,然而,酸性引起的n -基团质子化不利于它们与Fe离子配合形成高密度的Fe- n4位点。在这里,我们提出了一种质子化消除策略,将聚合产物浸泡在碱性溶液中以增加Fe-N4活性位点。理论计算表明,在pH为0和pH为7时,Fe离子与n基团结合反应的吉布斯自由能变化值分别为-3.70和-26.99 kcal/mol,表明去质子化有利于Fe- n配位。最终Fe-N-C催化剂的Fe-N4活性位点数量增加了两倍,表现出显著增强的ORR活性和优异的锌空气电池性能。这种去质子化效应可以应用于不同的胺类化合物和过渡金属离子,作为开发卓越的非贵金属碳催化剂的通用策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
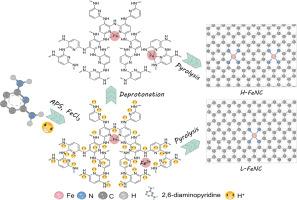
Deprotonation effect doubles active site density in Fe-N4-C catalyst for oxygen reduction electrocatalysis
Iron-nitrogen-carbon (Fe-N-C) materials with Fe-N4 structures have been considered as the most promising alternatives of scarce and precious platinum (Pt) for oxygen reduction reaction. Particularly, the high-temperature pyrolysis of a precursor mixture of N-containing amine polymers, Fe salts, and carbon supports, has become a popular method for the synthesis of high-performance Fe-N-C catalysts. The oxidative polymerization of amine monomers can usually proceed under acidic conditions, however, the acid-caused protonation of N-groups is not conducive to their coordination with Fe ions for the formation of high-density Fe-N4 sites. Here, we propose a protonation elimination strategy of soaking the polymerization products in alkaline solutions to increase Fe-N4 active sites. Theoretical calculations display that the Gibbs free energy change values of binding reactions between Fe ions and N-groups are -3.70 and -26.99 kcal/mol at pH 0 and 7, respectively, suggesting that the deprotonation can facilitate the Fe-N coordination. There is a two-fold increase in the number of Fe-N4 active sites for final Fe-N-C catalyst which exhibits significantly enhanced ORR activity and excellent Zn-air battery performance. This deprotonation effect can be applied to different amine compounds and transition-metal ions as a universal strategy for the development of preeminent non-precious metal carbon catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chinese Chemical Letters
化学-化学综合
CiteScore
14.10
自引率
15.40%
发文量
8969
审稿时长
1.6 months
期刊介绍:
Chinese Chemical Letters (CCL) (ISSN 1001-8417) was founded in July 1990. The journal publishes preliminary accounts in the whole field of chemistry, including inorganic chemistry, organic chemistry, analytical chemistry, physical chemistry, polymer chemistry, applied chemistry, etc.Chinese Chemical Letters does not accept articles previously published or scheduled to be published. To verify originality, your article may be checked by the originality detection service CrossCheck.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


