纳米MgO负载热敏HPCH@HA水凝胶通过骨免疫调节加速原位骨修复,同时促进血管生成和成骨
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
骨缺损修复是一个复杂的生理过程,始于炎症免疫系统的早期调节,涉及多种生理事件,包括血管生成、成骨分化和矿化。生物材料可以通过种植体-骨界面局部免疫微环境中的相关免疫细胞调节炎症反应,是再生医学领域的研究热点。目前,Mg2+调节骨微环境中的免疫细胞促进骨生成和血管生成主要集中在巨噬细胞上,而对T细胞的研究相对较少。同时,Mg2+的有效递送和释放仍然是一个挑战。为了解决这些问题,我们设计了一种新的热敏透明质酸-羟丙基几丁质水凝胶(HPCH@HA),它对Mg2+具有良好的亲和力并能持续释放。在体外,纳米MgO负载复合水凝胶可有效诱导巨噬细胞从M0表型向M2表型极化,同时激活T淋巴细胞,促进人脂肪源性干细胞(hADSCs)成骨分化矿化和人脐静脉内皮细胞(HUVECs)血管生成。在体内,在修复早期,复合水凝胶对小鼠颅骨关键缺损具有良好的修复效果。以上结果表明,我们设计的复合水凝胶可以在不使用外源细胞或诱导剂的情况下,有效调节骨组织免疫微环境,促进大骨缺损成熟骨的形成,支持原位骨再生。它是一种很有前途的用于骨组织工程的免疫调节生物材料。本文章由计算机程序翻译,如有差异,请以英文原文为准。
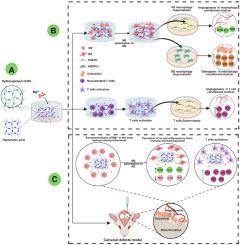
Nano MgO loaded thermosensitive HPCH@HA hydrogel accelerates in situ bone repair through osteoimmunomodulation while enhancing angiogenesis and osteogenesis
Bone defect repair is a complex physiological process, starting with early modulation by the inflammatory immune system, and involves multiple physiological events, including angiogenesis, osteogenic differentiation, and mineralization. Biomaterial can regulate inflammatory responses through relevant immune cells in the local immune microenvironment of the implant-bone interface which is a hot topic in the field of regenerative medicine. Currently, Mg2+ regulates immune cells in the bone microenvironment to promote osteogenesis and angiogenesis mainly focuses on macrophages,but there is relatively little research on T cells.At the same time, the effective delivery and release of Mg2+ remains a challenge. To address these issues, we designed a new thermosensitive hyaluronic acid-hydroxypropyl chitin hydrogel (HPCH@HA) that has good affinity for Mg2+ and can sustained release it. In vitro, nano MgO loaded complex hydrogels effectively induced macrophage polarization from M0 phenotype to M2 phenotype and simultaneously activate T lymphocytes which also promoted human adipose-derived stem cells (hADSCs) osteogenic differentiation and mineralization and human umbilical vein endothelial cells (HUVECs) angiogenesis. In vivo, at the early stage of repair, the composite hydrogel has a good repair effect on mouse skull critical defect. All these results show that our designed composite hydrogels can effectively regulate the immune microenvironment of bone tissue and promoting the formation of mature bone in large bone defects and supporting in situ bone regeneration without the use of exogenous cells or inducers. It's a promising candidate as immunomodulatory biomaterials for bone tissue engineering purposes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


