DNA甲基化抑制剂对光诱导的生物钟可塑性的影响
Q2 Medicine
引用次数: 0
摘要
下丘脑的视交叉上核(SCN)是一个主要的光反应生物钟,根据外部光信号的变化调节哺乳动物生理和行为的昼夜节律。尽管光如何重置昼夜节律的机制已经被表征,但光信号如何诱导昼夜节律周期的持续变化,即所谓的周期后效,仍然是难以捉摸的。本研究发现,在SCN附近应用DNA甲基转移酶抑制剂RG108可以阻断光周期变化对昼夜行为的周期后效。在单光脉冲作为生物钟重置刺激的水平上,RG108显著减弱了体内行为节律急性相移后的周期后效,并阻断了离体SCN中血管活性肠肽对生物钟基因节律的周期后效。此外,DNA甲基转移酶抑制剂SGI-1027阻断了光遗传神经元刺激对体外SCN节律的周期后效。然而,与随后的可塑性相比,急性时钟重置本身似乎不需要在SCN和行为水平上的DNA甲基化。我们的研究结果表明,DNA甲基化抑制剂在响应光周期和单光脉冲时可以阻断光诱导期的后效。先前的研究表明,SCN中的DNA甲基化对于非24小时光周期(t周期)的周期后效应至关重要,这表明SCN中的DNA甲基化可能是光诱导的昼夜节律周期可塑性的广泛机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
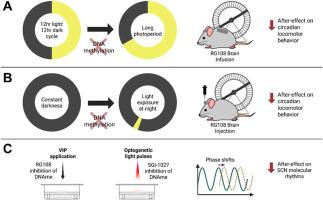
Effects of DNA methylation inhibitors on light-induced circadian clock plasticity
The suprachiasmatic nucleus (SCN) of the hypothalamus is a principal light-responsive circadian clock that adjusts circadian rhythms in mammalian physiology and behavior to changes in external light signals. Although mechanisms underlying how light acutely resets the timing of circadian rhythms have been characterized, it remains elusive how light signals induce lasting changes in circadian period, known as period after-effects. Here we have found that the period after-effects on circadian behavior of changing photoperiods are blocked by application of the DNA methyltransferase inhibitor RG108 near the SCN. At the level of single light pulses acting as clock-resetting stimulations, RG108 significantly attenuates period after-effects following acute phase shifts in behavioral rhythms in vivo, and blocks period after-effects on clock gene rhythms following phase resetting by the vasoactive intestinal peptide in the isolated ex vivo SCN. In addition, the DNA methyltransferase inhibitor SGI-1027 blocked period after-effects of optogenetic neuronal stimulation on ex vivo SCN rhythms. Acute clock resetting shifts themselves, however, do not appear to require DNA methylation at the SCN and behavioral levels, in contrast to subsequent period plasticity. Our results demonstrate that DNA methylation inhibitors block light-induced period after-effects in response to photoperiods and single light pulses. Together with previous studies showing that DNA methylation in the SCN is essential for period after-effects of non-24hr light cycles (T-cycles), this suggests that DNA methylation in the SCN may be a widespread mechanism of light-induced circadian period plasticity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Neurobiology of Sleep and Circadian Rhythms
Neuroscience-Behavioral Neuroscience
CiteScore
4.50
自引率
0.00%
发文量
9
审稿时长
69 days
期刊介绍:
Neurobiology of Sleep and Circadian Rhythms is a multidisciplinary journal for the publication of original research and review articles on basic and translational research into sleep and circadian rhythms. The journal focuses on topics covering the mechanisms of sleep/wake and circadian regulation from molecular to systems level, and on the functional consequences of sleep and circadian disruption. A key aim of the journal is the translation of basic research findings to understand and treat sleep and circadian disorders. Topics include, but are not limited to: Basic and translational research, Molecular mechanisms, Genetics and epigenetics, Inflammation and immunology, Memory and learning, Neurological and neurodegenerative diseases, Neuropsychopharmacology and neuroendocrinology, Behavioral sleep and circadian disorders, Shiftwork, Social jetlag.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


