靶向调节性T细胞活化的位点特异性聚乙二醇化白细胞介素-2减轻自身免疫性炎症
IF 3.6
Q2 IMMUNOLOGY
引用次数: 0
摘要
伴随着调节性T细胞(Treg)功能障碍的免疫稳态失调是各种自身免疫性和炎症性疾病的标志。虽然低剂量的白介素-2 (IL-2)治疗可以提高Treg水平并缓解疾病症状,但其半衰期短,需要频繁给药。此外,经常观察到与其他免疫细胞激活相关的不良事件。在这项研究中,利用位点特异性PEGylation方法,我们开发了一种新的IL-2变体I129-W80,它表现出il - 2r α -偏倚的结合谱,由PEG片段的空间位阻驱动。它在体外选择性激活Tregs,并能克服内源性诱饵受体可溶性IL-2Rα的抑制,不像fc融合IL-2变体AMG-592。在一项单剂量猴子研究中,I129-W80显示出延长的半衰期,同时持续扩增和激活Tregs。在不诱导c反应蛋白升高的最大剂量下,I129-W80的活性优于AMG-592。I129-W80改善了延迟型超敏反应和异种移植物抗宿主病模型的炎症反应。此外,在吡喹莫德诱导的皮炎模型中,与AMG-592相比,I129-W80在炎症组织中的分布减少。这些发现表明I129-W80相对于fc融合IL-2变体具有不同的特性,可以纠正Treg功能障碍引起的免疫失衡,从而改善各种自身免疫性疾病的症状。本文章由计算机程序翻译,如有差异,请以英文原文为准。
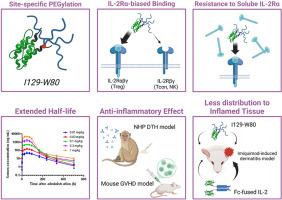
Targeted regulatory T cell activation by site-specific PEGylated interleukin-2 mitigates autoimmune inflammation
Dysregulation of immune homeostasis accompanied by regulatory T cell (Treg) dysfunction is a hallmark of various autoimmune and inflammatory diseases. While low-dose interleukin-2 (IL-2) treatment can enhance Treg levels and alleviate disease symptoms, its short half-life necessitates frequent dosing. Furthermore, adverse events associated with the activation of other immune cells are often observed. In this study, using a site-specific PEGylation approach, we developed a novel IL-2 variant, I129-W80, which exhibited an IL-2Rα–biased binding profile, driven by the steric hindrance of the PEG moiety. It selectively activated Tregs in vitro and could overcome inhibition by the endogenous decoy receptor, soluble IL-2Rα, unlike the Fc-fusion IL-2 variant AMG-592. In a single-dose monkey study, I129-W80 demonstrated an extended half-life, along with sustained amplification and activation of Tregs. At the maximum dose that did not induce C-reactive protein elevation, I129-W80 showed superior activity compared with AMG-592. I129-W80 improved inflammatory responses in both delayed-type hypersensitivity and xenogeneic graft-versus-host disease models. Additionally, in an imiquimod-induced dermatitis model, I129-W80 exhibited reduced distribution to inflamed tissues compared with AMG-592. These findings demonstrated that I129-W80 possesses distinct properties relative to Fc-fusion IL-2 variant and can correct immune imbalances caused by Treg dysfunction, thereby improving the symptoms of various autoimmune diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Translational Autoimmunity
Medicine-Immunology and Allergy
CiteScore
7.80
自引率
2.60%
发文量
33
审稿时长
55 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


