NNMT增强瘢痕疙瘩成纤维细胞侵袭和迁移能力及M2巨噬细胞极化。
IF 3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
瘢痕疙瘩的特征是成纤维细胞增生和过多的胶原沉积。烟酰胺n -甲基转移酶(Nicotinamide N-methyltransferase, NNMT)属于甲基转移酶家族,在包括瘢痕疙瘩形成在内的多种生理过程中发挥重要作用。然而,NNMT在瘢痕疙瘩发展中的作用仍然知之甚少。在这项研究中,我们通过博来霉素(BLM)诱导的体内纤维化模型和体外原代人瘢痕疙瘩成纤维细胞(HPKF)研究了NNMT在瘢痕疙瘩中的作用。瘢痕疙瘩组织中NNMT表达上调,瘢痕疙瘩组织中M2巨噬细胞显著增加。NNMT过表达降低了SAM含量,而NNMT敲低则增加了SAM含量。此外,过表达NNMT可增强HPKFs的细胞活力,而下调NNMT可促进细胞凋亡。此外,NNMT过表达促进HPKF的侵袭和迁移,而敲低则有效地抑制这些过程。NNMT过表达也增加了纤维化相关标志物的表达,包括FN1、COL1A1、COL3A1、Vimentin和α-SMA,而NNMT敲低则逆转了这一表达。用PMA将THP-1细胞诱导为THP1-M0巨噬细胞,并与HPKFs共培养。与过表达NNMT的HPKFs共培养可促进M0细胞中的M2极化,而NNMT敲低抑制M2极化,降低M0细胞中TGFB1和Arg-1的表达。此外,NNMT过表达促进了IGF-1的表达,用AG1024(一种IGF1R抑制剂)处理可以抑制NNMT诱导的M2极化,抑制IGF1B和Arg-1的表达。综上所述,我们的研究结果表明,NNMT增强了瘢痕疙瘩成纤维细胞的侵袭和迁移能力,并通过IGF-1调节瘢痕疙瘩中M2巨噬细胞的极化。这些结果突出了NNMT作为瘢痕疙瘩治疗的潜在治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
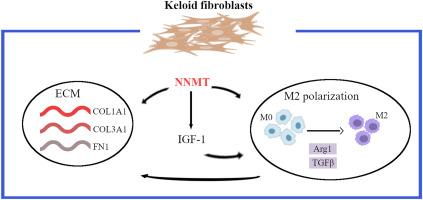
NNMT enhances invasive and migratory capacity of keloid fibroblasts and M2 macrophage polarization
Keloid are characterized by fibroblastic proliferation and excessive collagen deposition. Nicotinamide N-methyltransferase (NNMT) belongs to the methyltransferase family and plays an important role in various physiological processes, including keloid formation. However, the role of NNMT in keloid development remains poorly understood. In this study, we investigated the role of NNMT in keloids using a bleomycin (BLM)-induced fibrosis model in vivo and primary human keloid fibroblast (HPKF) in vitro. NNMT expression was upregulated in keloid tissues, and M2 macrophages were significantly increased in keloid tissue. NNMT overexpression reduced the SAM content, while NNMT knockdown increased it. Moreover, overexpression of NNMT enhanced the cell viability of HPKFs, while knockdown of NNMT promoted apoptosis. Additionally, NNMT overexpression promoted HPKF invasion and migration, whereas knockdown effectively inhibited these processes. Overexpression of NNMT also increased the expression of fibrosis-related markers, including FN1, COL1A1, COL3A1, Vimentin, and α-SMA, while NNMT knockdown reversed it. THP-1 cells were induced into THP1-M0 macrophages using PMA and co-cultured with HPKFs. Co-culturing with NNMT-overexpressing HPKFs promoted M2 polarization in M0 cells, while NNMT knockdown inhibited M2 polarization and reduced TGFB1 and Arg-1 expression in M0 cells. Furthermore, NNMT overexpression promoted IGF-1 expression, and treatment with AG1024 (an IGF1R inhibitor) suppressed NNMT-induced M2 polarization and inhibited IGF1B and Arg-1 expression. Taken together, our findings suggest that NNMT enhances the invasive and migratory capacity of keloid fibroblasts and regulates M2 macrophage polarization in keloids through IGF-1 modulation. These results highlight NNMT as a potential therapeutic target for keloid treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


