脲基苯磺酰胺醛糖还原酶和胆碱酯酶双重抑制剂的分子和结构表征。
IF 3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
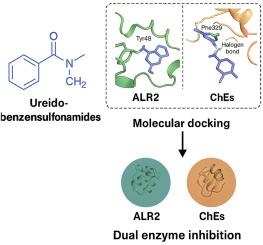
酶-配体相互作用的构象和动力学复杂性对于理解代谢和神经退行性病理的分子机制至关重要。在此,我们报道了一系列合理设计的脲基苯磺酰胺作为双作用纳米摩尔抑制剂,靶向醛糖还原酶(ALR2)和胆碱酯酶(AChE和BChE)。分光光度测定结果显示,化合物3SA-a对ALR2具有极强的亲和力(KI = 7.00±0.68 nM),比依帕司他高30倍以上。同样,3SA-f选择性抑制BChE的KI值为24.20±2.26 nM,是tacrine的7.8倍。分子对接模拟突出了不同的动态结合模式:3SA-a通过与Tyr48的氢键和水桥相互作用参与了ALR2的催化裂缝,而3SA-f利用了BChE扩展的酰基袋内的π-π堆叠和卤素键。这些结合方向与SAR结果一致,其中间磺酰胺放置和卤素取代优化了选择性和构象互补。互补的ADME-Tox预测证实了所有化合物的药物样性质(0 Lipinski/PAINS违反),中等口服渗透性(QPPCaco: 79-85 nm/s)和低中枢神经系统暴露(CNS评分= -2),与外周作用机制一致。总的来说,这项研究为双靶点酶抑制提供了一个详细的结构和动态框架,为未来针对ALR2-ChE轴的治疗提供了一个可调的支架。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Molecular and structural characterization of ureido-benzenesulfonamides as dual inhibitors of aldose reductase and cholinesterases
The conformational and kinetic intricacies of enzyme-ligand interactions are critical for understanding molecular mechanisms underlying metabolic and neurodegenerative pathologies. Herein, we report a rationally designed series of ureido-benzenesulfonamides as dual-acting nanomolar inhibitors targeting aldose reductase (ALR2) and cholinesterases (AChE and BChE). Spectrophotometric inhibition assays revealed that compound 3SA-a displayed exceptional ALR2 affinity (KI = 7.00 ± 0.68 nM), surpassing epalrestat by over 30-fold. Likewise, 3SA-f selectively inhibited BChE with a KI of 24.20 ± 2.26 nM, outperforming tacrine by a factor of 7.8. Molecular docking simulations highlighted distinct dynamic binding modes: 3SA-a engaged ALR2's catalytic cleft through a hydrogen bond with Tyr48 and water-bridged interactions, whereas 3SA-f leveraged π-π stacking and halogen bonding within BChE's extended acyl pocket. These binding orientations were consistent with SAR findings, where meta-sulfonamide placement and halogen substitution optimized selectivity and conformational complementarity. Complementary in silico ADME-Tox predictions confirmed the drug-like nature of all compounds (0 Lipinski/PAINS violations), moderate oral permeability (QPPCaco: 79–85 nm/s), and low CNS exposure (CNS score = −2), aligning with a peripheral mechanism of action. Collectively, this study provides a detailed structural and dynamic framework for dual-target enzyme inhibition, offering a tunable scaffold for future therapeutics targeting the ALR2-ChE axis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


