越界基因表达破坏了两种入侵白蚁杂交的蜕皮过程。
IF 3.7
2区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
台湾地下白蚁(Coptotermes formosanus)和亚洲地下白蚁(Coptotermes gestroi)是世界上最具破坏性的白蚁害虫。这两个物种已经扩散到世界各地,在一些地区重叠分布,它们可以潜在地杂交。观察表明,杂交群体中的工蜂蜕皮速度比亲本物种慢,这表明蜕皮过程的中断是杂交不相容的一种形式。我们的目标是鉴定在蜕皮过程中杂种中错误表达的基因,以帮助揭示蜕皮中断的分子机制。我们进行了rna测序,并通过对差异表达转录物进行时间过程分析来鉴定蜕皮相关基因。我们在亲本物种的蜕皮周期的每个阶段(蜕皮前、蜕皮后和蜕皮间)鉴定了蜕皮相关基因。然后,我们比较了这些基因在杂交种中的表达水平,以确定与亲本物种相比,过度表达(或过表达或过表达)的基因。我们发现了几个与蜕皮周期、肌肉收缩、应激反应和蜕皮激素代谢相关的基因,这些基因在杂交后代中相对于亲代表达不足。这些差异可能有助于解释杂交种蜕皮的中断,并为杂交对生长发育关键时期基因错误表达的影响提供见解。此外,通过对地下白蚁蜕皮相关基因的鉴定,揭示了地下白蚁这种具有高度发育可塑性的昆虫蜕皮过程的分子途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
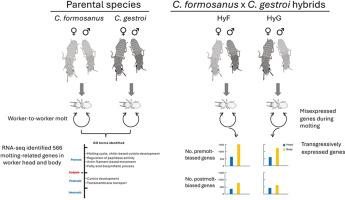
Transgressive gene expression disrupts the molting process in hybrids of two invasive termites
The Formosan subterranean termite (Coptotermes formosanus) and the Asian subterranean termite (Coptotermes gestroi) are among the most destructive termite pests in the world. Both species have spread to various regions worldwide with overlapping distributions in a few areas where they can potentially hybridize. Observations suggest that workers in hybrid colonies are slower to molt than those of the parental species, suggesting a disruption in the molting process as a form of hybrid incompatibility. Our goal was to identify misexpressed genes in hybrids during the molting process to help uncover the molecular mechanisms underlying molting disruption. We conducted RNA-seq and identified molting-related genes by performing a time course analysis on differentially expressed transcripts. We identified molting-related genes during each stage of the molting cycle (pre-, post- and inter-molt) in the parental species. We then compared expression levels of these genes in the hybrids to identify genes that were transgressively expressed (either over- or under-expressed) compared to the parental species. We identified several genes related to the molting cycle, muscle contraction, response to stress, and ecdysone metabolism that were under-expressed in hybrids relative to their parents. These differences may help explain the disruption of molting in hybrids and provide insights into the effects of hybridization on misexpression of genes during critical periods of growth and development. Moreover, identification of molting related genes in subterranean termites highlights the molecular pathways involved in the molting process in this group of insects with high developmental plasticity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.40
自引率
5.30%
发文量
105
审稿时长
40 days
期刊介绍:
This international journal publishes original contributions and mini-reviews in the fields of insect biochemistry and insect molecular biology. Main areas of interest are neurochemistry, hormone and pheromone biochemistry, enzymes and metabolism, hormone action and gene regulation, gene characterization and structure, pharmacology, immunology and cell and tissue culture. Papers on the biochemistry and molecular biology of other groups of arthropods are published if of general interest to the readership. Technique papers will be considered for publication if they significantly advance the field of insect biochemistry and molecular biology in the opinion of the Editors and Editorial Board.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


