利用Ni粒子和Ni - ceria界面之间的协同作用实现高效的生物质还原胺化
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
5-羟甲基糠醛(HMF)催化还原胺化制伯胺已成为一种很有前途的纤维素生物质升级途径。巧妙地将不同的催化中心整合在单一催化剂上,可以协同驱动有机反应物和H2分子的转化,控制复杂胺化过程中的产物选择性。本文报道了一种具有Ni颗粒表面和Ni - ceo2界面双功能Ni位点的铈负载Ni纳米催化剂。调节Ni颗粒的大小可以逐渐调节Ni与ceria的相互作用。因此,可以很好地调节Ni0活性位点的暴露量和Ni在不同位置的催化功能。设计的Ni催化剂在100℃下,在2bar的低压H2压力下,获得了100%的5-氨基甲基-2-呋喃醇(AMF)收率和高的生成速率(10.6 gAMF−1 gNi−1 h−1)。通过对Ni尺寸效应和反应参数的研究,揭示了产物分布的显著差异,从而对复杂的反应路径有了更深入的了解。进一步选择性转化反应中间体(即亚胺和希夫碱)对于抑制副产物(即三聚体和糠醛)和提高AMF收率至关重要。物理表征和先进的化学吸附技术表明,希夫碱和H2分别发生在Ni表面和Ni - ceo2界面上的吸附和活化。氨解过程涉及NH3活化,发生在Ni2+位点上。所设计的Ni/CeO2催化剂对多种生物胺的合成具有良好的底物范围,并且经过适当的再生后具有稳定的可重复使用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
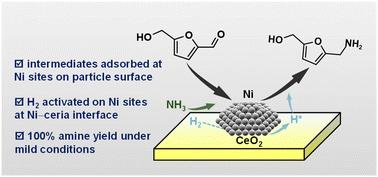
Harnessing the synergism between Ni particles and an Ni–ceria interface for efficient biomass reductive amination
Catalytic reductive amination of 5-hydroxymethylfurfural (HMF) into primary amine has become a very promising upgrading route for cellulosic biomass. Ingenious integration of distinct catalytic centers on a single catalyst can synergistically drive the transformation of organic reactants and H2 molecules, controlling product selectivity in this complex amination process. Herein, a ceria-supported Ni nanocatalyst with bifunctional Ni sites from the Ni particle surface and Ni–CeO2 interface is reported. Regulating the size of Ni particles can gradually tune the interaction between Ni and ceria. Thus, the exposed amount of Ni0 active sites and the catalytic function of Ni at distinct locations can be finely modulated. The designed Ni catalyst achieved 100% yield of 5-aminomethyl-2-furanyl alcohol (AMF) and a high formation rate (10.6 gAMF−1 gNi−1 h−1) at 100 °C under a hypobaric H2 pressure of only 2 bar. The investigation of the Ni size effect and reaction parameters revealed significant differences in product distribution, enabling a deeper understanding of the complex reaction path. It was disclosed that further selective transformations of reaction intermediates (i.e., imine and Schiff bases) are crucial for inhibiting byproducts (i.e., trimers and furfurine) and increasing the AMF yield. Physical characterizations and advanced chemisorption techniques revealed that the adsorption and activation of the Schiff base and H2 occurred at the Ni surface and Ni–CeO2 interface, respectively. The ammonolysis process involving NH3 activation occurred on Ni2+ sites. The designed Ni/CeO2 catalyst demonstrated good substrate scope for various bio-amine syntheses and exhibited stable reusability after suitable regeneration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


