使用计算流体动力学的粒子沉积特定主题建模框架
IF 2.9
3区 环境科学与生态学
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
量化呼吸道中的颗粒沉积和剂量需要生理上真实的表示和可重复的计算工作流程。然而,现有的建模框架,如国际放射防护委员会(ICRP)的区室模型和多路径粒子剂量学(MPPD)工具,缺乏详细的沉积剖面和特定主题的能力。应用于呼吸道的计算机视觉算法和计算流体与粒子动力学(CFPD)的进步相结合,可以在个体CT扫描中获得解剖学精确几何形状的粒子行为的高保真模拟。CFPD模拟的分割、预处理和文件准备任务通常是耗时的,迄今为止还没有先前的研究提出一个完全自动化的框架。这项工作提出了一个完全自动化的工作流程,以获得个性化的颗粒沉积剖面在人的呼吸道。该管道首先使用形态学和基于深度学习的方法分割上下气道几何形状,从CT成像数据生成三维(3D)模型。接下来,提出了一系列算法来进行质量检查并为CFD或CFPD模拟准备3D几何形状。预处理步骤包括校正几何伪影,强制物理一致的网格,以及自动识别和限制多个出口,这些都是CFD/CFPD模拟所需的。然后将这些处理过的模型输入到开源(OpenFOAM)或商用(StarCCM+) CFD求解器中,在这些求解器中,在实际呼吸条件下求解流动和瞬态粒子输运方程,包括湍流和粒子-壁相互作用。最后,所得的粒子沉积剖面可以与蒙特卡罗辐射传输代码和最先进的计算模型相结合,以评估放射性气溶胶吸入情况下器官特异性吸收剂量。提出的工作简化了呼吸道分割、CFD/CFPD模拟的预处理以及与剂量评估工作流程的集成,减少了人工干预,并改善了高保真度、特定受试者建模的访问。预测颗粒沉积和剂量分布的高精度可以改善呼吸医学的个性化治疗策略,并改进辐射防护的剂量估计。本文章由计算机程序翻译,如有差异,请以英文原文为准。
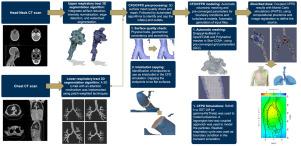
Subject-specific modeling framework for particle deposition using computational fluid dynamics
Quantifying particle deposition and dose in the respiratory tract requires a physiologically realistic representation and reproducible computational workflows. However, existing modeling frameworks, such as the International Commission on Radiological Protection (ICRP) compartmental models and the Multiple Path Particle Dosimetry (MPPD) tool, lack detailed deposition profiles and subject-specific capabilities. The combination of advances in computer vision algorithms applied to the respiratory tract and Computational Fluid and Particle Dynamics (CFPD) allows high-fidelity simulations of particle behavior in anatomically accurate geometries derived from individual CT scans. The segmentation, preprocessing, and file preparation task for a CFPD simulation was often time-consuming, and no prior studies to-date have yet presented a fully automated framework.
This work presents a fully automated workflow to obtain individualized particle deposition profiles in the human respiratory tract. The pipeline starts with segmenting upper and lower airway geometries using morphological and deep learning-based methods, generating three-dimensional (3D) models from CT imaging data. Next, a series of algorithms are presented to quality check and prepare the 3D geometry for a CFD or CFPD simulation. The preprocessing step includes correcting geometric artifacts, enforcing a physically consistent mesh, and automatically identifying and capping multiple outlets, which is required for CFD/CFPD simulations. These processed models are then input into open-source (OpenFOAM) or commercial (StarCCM+) CFD solvers, where flow and transient particle transport equations — including turbulence and particle–wall interactions are solved under realistic breathing conditions. Finally, the resulting particle deposition profiles can be integrated with Monte Carlo radiation transport codes and state-of-the-art computational phantoms to assess organ-specific absorbed doses in scenarios of radioactive aerosol inhalation.
The presented work streamlines respiratory tract segmentation, preprocessing for CFD/CFPD simulations, and integration with dose assessment workflows, reducing manual intervention and improving access to high-fidelity, subject-specific modeling. The high precision in predicted particle deposition and dose distributions can improve personalized treatment strategies in respiratory medicine and refine dose estimates for radiation protection.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Aerosol Science
环境科学-工程:化工
CiteScore
8.80
自引率
8.90%
发文量
127
审稿时长
35 days
期刊介绍:
Founded in 1970, the Journal of Aerosol Science considers itself the prime vehicle for the publication of original work as well as reviews related to fundamental and applied aerosol research, as well as aerosol instrumentation. Its content is directed at scientists working in engineering disciplines, as well as physics, chemistry, and environmental sciences.
The editors welcome submissions of papers describing recent experimental, numerical, and theoretical research related to the following topics:
1. Fundamental Aerosol Science.
2. Applied Aerosol Science.
3. Instrumentation & Measurement Methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


