基于crispr的SARS-CoV-2及其新变体和多种病原体的分子检测
IF 1.8
4区 医学
Q3 INFECTIOUS DISEASES
Diagnostic microbiology and infectious disease
Pub Date : 2025-08-12
DOI:10.1016/j.diagmicrobio.2025.117062
引用次数: 0
摘要
SARS-CoV-2 (SCoV-2)等致病性病毒继续对人类文明构成重大威胁。从SCoV-2感染中吸取的教训突出表明,需要强大且易于获得的诊断工具,以限制病毒传播并预防COVID-19等未来的大流行。基于rt - qpcr的检测通常用于敏感和准确的诊断,这需要复杂的仪器,实验室设置和技术专长。虽然RT-qPCR是高度可靠的,被认为是病原体检测的金标准,但它昂贵、耗时,而且对大众来说负担不起。因此,需要其他可靠的、灵敏度、特异性和准确性与RT-qPCR相当的基于核酸的检测方法。CRISPR技术的最新进展承诺其作为POC测试设备的发展,提供高端,无仪器,便携式和经济高效的工作流程。此外,COVID-19大流行鼓励了下一代基于crispr的诊断技术的发展,并提供了家庭检测的规定,这导致了便携式和智能手机集成手持设备的发展,这些设备可以在比RT-qPCR更短的时间内检测出各种致病性感染。对于诊断SCoV-2的存在,基于crispr的诊断(SHERLOCK/DETECTR)比RT-qPCR(90-180分钟;10-50美元/次)显示出等效的特异性(100%)和接近等效的灵敏度(基于crispr的诊断为93-100%,而RT-qPCR为95-100%)。对于高灵敏度的集中检测,RT-qPCR仍然是黄金标准,但CRISPR在护理点环境下工作得很好,因为它只需要很少的设备(如侧流条带或加热块),并且允许多路复用。像CARMEN这样基于crispr的诊断突破性平台利用微流体技术在一次运行中测试5000多个样本,不像RT-qPCR,需要对每个目标进行单独的反应。本文综述了CRISPR技术的进展,如SHERLOCK、DETECTR和其他基于cas -9的诊断方法,重点介绍了基于CRISPR的诊断方法检测SCoV-2及其新出现的voc,并强调了它们与金标准RT-qPCR相比的优势和局限性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
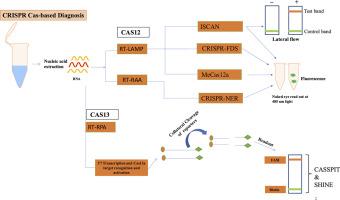
CRISPR-based molecular detection of SARS-CoV-2, its emerging variants, and diverse pathogens
Pathogenic viruses such as SARS-CoV-2 (SCoV-2), continue to pose a significant threat to human civilization. The lessons learnt from SCoV-2 infections have highlighted the requirement for robust and readily available diagnostic tools in order to limit the virus transmission and prevent future pandemics such as COVID-19. RT-qPCR-based detection is routinely used for sensitive and accurate diagnosis, which requires a sophisticated instrument, laboratory setup, and technical expertise. Though RT-qPCR is highly reliable and considered the gold standard for pathogen detection, it is costly, time-consuming, and unaffordable for the masses. Therefore, other reliable methods for nucleic acid-based detection with sensitivity, specificity, and accuracy on-par with RT-qPCR are required. Recent advancement in CRISPR technology promises its development as a POC testing device, providing a high-end, instrument-free, portable, and cost-effective workflow. Further, COVID-19 pandemic has encouraged the development of next-generation CRISPR-based diagnostics with a provision for home-testing which has resulted in the development of portable and smart-phone integrated hand-held devices which can detect various pathogenic infections in a shorter time frame than RT-qPCR. For diagnosing the presence of SCoV-2, CRISPR-based diagnostics (SHERLOCK/DETECTR) are quicker (30–60 min), less expensive ($5–15/test), and portable than RT-qPCR (90–180 min; $10–50/test) demonstrating equivalent specificity (100%) and near-equivalent sensitivity (93-100% for CRISPR-based diagnostics vs 95-100% for RT-qPCR). For high-sensitivity centralized testing, RT-qPCR is still the gold standard, but CRISPR works well in point-of-care settings because it requires little equipment (like lateral flow strips or heating blocks) and allows multiplexing. CRISPR-based diagnostics breakthrough platform like CARMEN leverages microfluidic technology to test 5,000 plus samples in a single run, unlike RT-qPCR, which requires separate reactions for each target.In this review, the advancement in CRISPR technology such as SHERLOCK, DETECTR, and other Cas-9-based diagnostics are highlighted which exclusively focuses on the CRISPR-based diagnostics to detect SCoV-2 and its emerging VOCs, highlighting their advantages and limitations compared to the gold-standard RT-qPCR.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.30
自引率
3.40%
发文量
149
审稿时长
56 days
期刊介绍:
Diagnostic Microbiology and Infectious Disease keeps you informed of the latest developments in clinical microbiology and the diagnosis and treatment of infectious diseases. Packed with rigorously peer-reviewed articles and studies in bacteriology, immunology, immunoserology, infectious diseases, mycology, parasitology, and virology, the journal examines new procedures, unusual cases, controversial issues, and important new literature. Diagnostic Microbiology and Infectious Disease distinguished independent editorial board, consisting of experts from many medical specialties, ensures you extensive and authoritative coverage.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


