自组装的混合水凝胶微球为骨骼再生创造了一个模拟骨髓的生态位
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
骨髓(Bone marrow, BM)是一种富含生长因子和骨髓间充质干细胞(Bone marrow mesenchymal stem cells, BMSCs)的天然生态位,提供了最佳的再生微环境,被广泛应用于临床。然而,骨髓间充质干细胞有限的增殖能力和骨再生与生长因子释放之间的不匹配限制了其在治疗严重骨缺损方面的有效性。从BM的再生特性中获得灵感,我们开发了自组装混合微球来复制其功能,并通过组织工程方法解决这些挑战。这个模拟骨髓基质的生态位通过快速降解明胶甲基丙烯酰(GelMA)微球富集骨髓间充质干细胞,该微球装载外源骨髓间充质干细胞并与干细胞归巢肽(SKP)偶联以募集内源性骨髓间充质干细胞。SKP进一步增强骨髓间充质干细胞的干性,从而促进血管生成和消除炎症。缓慢降解的壳聚糖甲基丙烯酰微球促进血管生成(KLT)和成骨(OGP)肽的持续释放,支持血管成熟和成骨。BMSCs和SKP的早期释放,随后OGP和KLT的释放,与骨再生的动态过程一致。在大鼠股骨髁严重缺损模型中,模拟脑损伤的生态位形成原位骨化中心,显著促进骨再生。本研究介绍了一种新的骨髓基质模拟生态位,其特征是骨髓间充质干细胞富集的环境和治疗因子的顺序释放,为治疗严重骨缺损提供了一种有希望的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
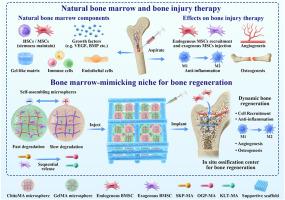
Self-assembled hybrid hydrogel microspheres create a bone marrow-mimicking niche for bone regeneration
Bone marrow (BM), a natural niche rich in growth factors and bone marrow mesenchymal stem cells (BMSCs), provides an optimal regenerative microenvironment and is widely used in clinical applications. However, the limited proliferative capacity of BMSCs and the mismatch between bone regeneration and growth factors release constrain their effectiveness in treating critical bone defects. Drawing inspiration from the regenerative properties of BM, we developed self-assembled hybrid microspheres to replicate its function and address these challenges through a tissue engineering approach. This BM-mimicking niche enriched BMSCs via fast-degrading gelatin methacryloyl (GelMA) microspheres, which were loaded with exogenous BMSCs and conjugated with stem cell homing peptides (SKP) to recruit endogenous BMSCs. SKP further enhanced the stemness of BMSCs, thereby promoting angiogenesis and resolving inflammation. Slow-degrading chitosan methacryloyl (ChitoMA) microspheres facilitated sustained release of angiogenic (KLT) and osteogenic (OGP) peptides, supporting blood vessel maturation and osteogenesis. The early release of BMSCs and SKP, followed by the subsequent release of OGP and KLT, aligned with the dynamic process of bone regeneration. In a rat critical femoral condyle defect model, the BM-mimicking niche formed an in-situ ossification center, significantly enhancing bone regeneration. This study introduces a novel BM-mimicking niche characterized by a BMSC-enriched environment and the sequential release of therapeutic factors, offering a promising strategy for treating critical bone defects.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


