通过可逆Aza-Michael加成的聚合物网络闭环化学回收
IF 5.1
3区 工程技术
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
以2,6-二叔丁基-7-苯基-对醌甲基(pQM)与仲胺基进行可逆aza-Michael加成反应,制备了一种新型闭环可回收聚合物网络材料。采用高效的aza-Michael加成反应将端基为pQM的双官能单体与三[2-(甲氨基)乙基]胺(TMEN)三官能交联,可在室温条件下方便地制备聚合物网络。由于其可逆反应的性质,二碳酸二叔丁酯可以很容易地降解交联聚合物网络,以捕获释放的仲胺基,从而刺激反向aza-Michael加成反应。通过这种降解方法,pQM单体和TMEN交联剂的回收率分别达到85%和75%以上。通过简单地操纵pQM单体的化学结构,合成的聚合物网络材料的热性能和机械性能可以灵活地调整,范围从弹性体到塑料。本文章由计算机程序翻译,如有差异,请以英文原文为准。
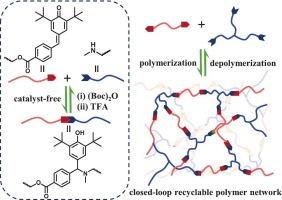
Closed-loop chemical recycling of polymer networks via reversible Aza-Michael addition
A new type of closed-loop recyclable polymer network material was developed based on a reversible aza-Michael addition reaction between 2,6-di-t-butyl-7-phenyl-p-quinone methide (pQM) and secondary amine group. The polymer networks could be conveniently prepared at ambient conditions using the efficient aza-Michael addition to crosslink the difunctional monomers with pQM end groups and trifunctional crosslinker of tris[2-(methylamino)ethyl]amine (TMEN). By virtue of the reversible reaction property, the crosslinked polymer networks could be easily degraded using di-tert-butyl dicarbonate to trap the released secondary amine groups to stimulate the reverse aza-Michael addition reaction. The pQM monomers and TMEN crosslinkers could be recovered with high yields above 85 % and 75 % by this degradation approach, respectively. The thermal and mechanical properties of the resultant polymer network materials could be flexibly tuned ranging from elastomers to plastics, by simply manipulating the chemical structure of the pQM monomers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Reactive & Functional Polymers
工程技术-高分子科学
CiteScore
8.90
自引率
5.90%
发文量
259
审稿时长
27 days
期刊介绍:
Reactive & Functional Polymers provides a forum to disseminate original ideas, concepts and developments in the science and technology of polymers with functional groups, which impart specific chemical reactivity or physical, chemical, structural, biological, and pharmacological functionality. The scope covers organic polymers, acting for instance as reagents, catalysts, templates, ion-exchangers, selective sorbents, chelating or antimicrobial agents, drug carriers, sensors, membranes, and hydrogels. This also includes reactive cross-linkable prepolymers and high-performance thermosetting polymers, natural or degradable polymers, conducting polymers, and porous polymers.
Original research articles must contain thorough molecular and material characterization data on synthesis of the above polymers in combination with their applications. Applications include but are not limited to catalysis, water or effluent treatment, separations and recovery, electronics and information storage, energy conversion, encapsulation, or adhesion.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


