利用原位制备的Cu0@ACF吸附剂高效去除溶液中的放射性碘
IF 8
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
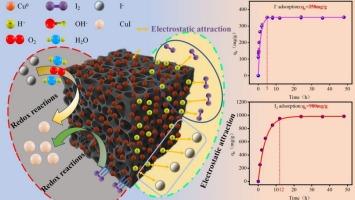
快速和有效地从核事故和废水废物中去除放射性碘对于保护生活环境和维持人类健康至关重要。采用原位自还原法制备了纳米零价铜改性活性炭纤维(Cu0@ACF)复合材料。采用x射线衍射(XRD)和x射线光电子能谱(XPS)系统分析了原始样品和碘吸附样品(ACF和Cu0@ACF)的结构和化学成分。利用能量色散光谱(EDS)验证了碳和铜的共存。通过批量实验全面考察了改性剂浓度、接触时间、pH值和干扰离子对I2和I−去除率的影响。4 wt% Cu0@ACF对I2的吸附量为980 mg/g (C0 = 1000 mg/L, pH = 2),对I -的吸附量为358 mg/g (C0 = 400 mg/L, pH = 2),明显优于其他报道的碘吸附剂。动力学研究表明,吸附过程遵循准二级动力学,等温线数据最好用Langmuir模型(R2 >;0.99),表明单层化学吸附。吸附机制主要是碘离子(I−)与锚定在ACF表面的金属纳米铜(Cu0)相互作用,形成以极性共价键为特征的CuI。总之,Cu0@ACF在放射性废水处理应用方面具有突出的潜力,这项工作为开发高性能acf基吸附剂提供了一个简单的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Highly efficient removal of radioactive iodine from solution using in situ prepared Cu0@ACF adsorbent
Rapid and efficient removal of radioactive iodine from nuclear accidents and effluent waste was significantly essential to protect the living environment and maintain human health. The nano-zero-valent copper modified activated carbon fiber (Cu0@ACF) composite was successfully synthesized via an in situ self-reduction method. The structural and chemical compositions of both pristine and iodine-adsorbed samples (ACF and Cu0@ACF) were systematically analyzed by X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS). The coexistence of carbon and copper was verified using energy dispersive spectroscopy (EDS). The effects of modifier concentration, contact time, pH value and interfering ions on the removal of I2 and I− were comprehensively investigated through batch experiments. The adsorption capacity of 4 wt% Cu0@ACF for I2 reached 980 mg/g (C0 = 1000 mg/L, pH = 2), and for I− was 358 mg/g (C0 = 400 mg/L, pH = 2), significantly outperforming other reported iodine adsorbents. The kinetic studies revealed that the adsorption processes followed pseudo-second-order kinetics, while isotherm data were best described by the Langmuir model (R2 > 0.99), indicating monolayer chemisorption. The adsorption mechanism was dominated by chemical reactions where in iodide ions (I−) interact with the metallic nano‑copper (Cu0) anchored on the ACF surface, resulting in the formation of CuI characterized by polar covalent bonding. In a word, Cu0@ACF showed an outstanding potential for radioactive wastewater treatment applications and this work presents a facile strategy for developing high-performance ACF-based adsorbents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science of the Total Environment
环境科学-环境科学
CiteScore
17.60
自引率
10.20%
发文量
8726
审稿时长
2.4 months
期刊介绍:
The Science of the Total Environment is an international journal dedicated to scientific research on the environment and its interaction with humanity. It covers a wide range of disciplines and seeks to publish innovative, hypothesis-driven, and impactful research that explores the entire environment, including the atmosphere, lithosphere, hydrosphere, biosphere, and anthroposphere.
The journal's updated Aims & Scope emphasizes the importance of interdisciplinary environmental research with broad impact. Priority is given to studies that advance fundamental understanding and explore the interconnectedness of multiple environmental spheres. Field studies are preferred, while laboratory experiments must demonstrate significant methodological advancements or mechanistic insights with direct relevance to the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


