小檗碱通过增加嗜粘阿克曼氏菌的丰度来减轻代谢功能障碍相关的脂肪性肝炎。
IF 4.9
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
代谢功能障碍相关脂肪性肝炎(MASH)与肠道屏障缺陷和肠道微生物群失调有关。肠道共生细菌Akkermansia muciniphila (Akk)维持肠道屏障完整性并改善mash相关代谢综合征。小檗碱(BBR)是一种传统的中药,在治疗MASH方面表现出希望。然而,针对Akk调控及其潜在机制的药物研究仍然有限。本研究探讨了BBR在MASH中调控Akk的机制。我们给C57BL/6J雄性小鼠喂食蛋氨酸胆碱缺乏(methionine-choline-deficient, MCD) 6周,建立小鼠MASH模型。采用16S rRNA测序分析肠道菌群,qPCR测定细菌数量。应用抗生素鸡尾酒(Abx)和粪便菌群移植(FMT)调节肠道菌群。结果表明,BBR可减轻肝脏和结肠炎症,保持肠道屏障完整性,防止微生物群转移到肝脏。16S rRNA测序和qPCR分析显示,BBR处理后粪便样品中Akk丰度显著增加。机制上,BBR不直接促进Akk的生长,但减少细菌负荷,提高MUC2表达,从而间接促进了Akk的定植。虽然抗生素治疗对肠道微生物群的破坏削弱了小檗碱对MASH的治疗作用,但移植bbr处理小鼠的粪便微生物群可以减轻抗生素处理小鼠的MASH。最后,BBR和Akk对MASH表现出协同治疗作用。我们的研究表明,BBR通过提高Akk丰度和恢复肠道屏障完整性来缓解MASH小鼠。BBR和Akk联合治疗将是预防MASH的有希望的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
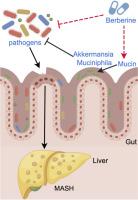
Berberine alleviates metabolic dysfunction-associated steatohepatitis by enhancing the abundance of Akkermansia muciniphila
Metabolic dysfunction-associated steatohepatitis (MASH) is associated with intestinal barrier defects and gut microbiota dysbiosis. The gut commensal bacterium Akkermansia muciniphila (Akk) maintains intestinal barrier integrity and improves MASH-related metabolic syndromes. Berberine (BBR), a traditional Chinese medicine, shows promise in treating MASH. However, research on drugs that target Akk regulation and its underlying mechanisms remains limited. This study investigates the mechanisms by which BBR regulates Akk in MASH. We fed C57BL/6 J male mice a methionine-choline-deficient (MCD) diet for 6 weeks to establish the MASH mouse models. The gut microbiota was analyzed using 16S rRNA sequencing and bacterial quantification measured by qPCR analysis. An antibiotic cocktail (Abx) and fecal microbiota transplantation (FMT) were applied to modulate gut microbiota. Results showed that BBR reduced hepatic and colonic inflammation, preserved intestinal barrier integrity and prevented microbiota translocation into the liver. The 16S rRNA sequencing and qPCR analysis revealed a significant increase in Akk abundance in fecal samples following BBR treatment. Mechanistically, BBR did not promote Akk growth directly, but it reduced the bacterial load and enhanced MUC2 expression, thereby facilitating Akk colonization indirectly. While disruption of the gut microbiota by antibiotics treatment weakened the therapeutic effect of berberine on MASH, transplanting of the fecal microbiota from BBR-treated mice could mitigate MASH in antibiotic-treated mice. Finally, BBR and Akk exhibited synergistic therapeutic effects against MASH. Our study illustrated that BBR alleviates MASH mice by enhancing Akk abundance and restoring intestinal barrier integrity. BBR and Akk combination therapy would be a promising strategy for MASH prevention.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Nutritional Biochemistry
医学-生化与分子生物学
CiteScore
9.50
自引率
3.60%
发文量
237
审稿时长
68 days
期刊介绍:
Devoted to advancements in nutritional sciences, The Journal of Nutritional Biochemistry presents experimental nutrition research as it relates to: biochemistry, molecular biology, toxicology, or physiology.
Rigorous reviews by an international editorial board of distinguished scientists ensure publication of the most current and key research being conducted in nutrition at the cellular, animal and human level. In addition to its monthly features of critical reviews and research articles, The Journal of Nutritional Biochemistry also periodically publishes emerging issues, experimental methods, and other types of articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


