有丝分裂定位信号(Mitosis Localization Signal, MLS)在爪蟾胚胎中延伸KA1,调控MELK激酶在质膜上的定位和活性。
IF 2.1
3区 生物学
Q2 DEVELOPMENTAL BIOLOGY
引用次数: 0
摘要
MELK是一种依赖于细胞周期的丝氨酸/苏氨酸蛋白激酶,其表达在增殖细胞和癌细胞中升高。在爪蟾胚胎中,MELK过表达诱导细胞分裂失败,导致多核细胞。这种表型需要MELK的催化活性,这与MELK的构象修饰及其在细胞膜上的定位有关。MELK的激活和定位是如何协调的尚不清楚。在这里,我们发现在非洲爪蟾原肠上皮细胞中,MELK在质膜上突然富集,从中期到后期过渡到早期间期。我们发现,删除与阴离子磷脂结合的激酶相关结构域1 (KA1)并不会取消MELK在质膜上的定位。通过一系列的缺失,我们发现了一个新的41个氨基酸结构域,称为有丝分裂定位信号(MLS),它调节分裂细胞中MELK向质膜的定位。我们发现MLS与KA1协同调节MELK的定位,并且在MELK过表达时诱导细胞分裂失败是必要的。我们的研究结果强调了MLS在MELK定位和调节MELK活性中的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
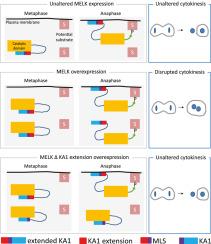
Mitosis Localization Signal (MLS) extends KA1 and regulates MELK kinase localization to plasma membrane and activity in Xenopus embryo
MELK is a cell-cycle dependent serine/threonine protein kinase whose expression is elevated in proliferating and cancer cells. In the Xenopus embryo, MELK overexpression induces cytokinesis failure, leading to multinucleated cells. This phenotype requires MELK catalytic activity, which correlates with MELK conformational modification and its localization to the cell membrane. How MELK activation and localization are coordinated remains unclear. Here, we show that in Xenopus gastrula epithelial cells MELK is abruptly enriched at the plasma membrane starting precisely from the metaphase-to-anaphase transition until early interphase. We show that deletion of the Kinase-Associated domain 1 (KA1), involved in binding to anionic phospholipids, does not abolish MELK localization to the plasma membrane. By a series of deletions, we identified a new 41-amino-acid domain, called Mitosis Localization Signal (MLS) that regulates MELK localization to the plasma membrane in dividing cells. We show that MLS cooperates with KA1 to regulate MELK localization and is necessary to induce cytokinesis failure when MELK is overexpressed. Our findings highlight the importance of MLS in MELK localization and in regulating MELK activity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Developmental biology
生物-发育生物学
CiteScore
5.30
自引率
3.70%
发文量
182
审稿时长
1.5 months
期刊介绍:
Developmental Biology (DB) publishes original research on mechanisms of development, differentiation, and growth in animals and plants at the molecular, cellular, genetic and evolutionary levels. Areas of particular emphasis include transcriptional control mechanisms, embryonic patterning, cell-cell interactions, growth factors and signal transduction, and regulatory hierarchies in developing plants and animals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


