小鼠β冠状病毒MHV-RSA59在神经炎症中协调氧化应激和炎症途径的相互作用
IF 2.4
3区 医学
Q3 VIROLOGY
引用次数: 0
摘要
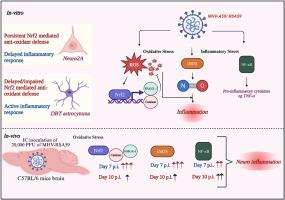
小鼠肝炎病毒MHV-A59/RSA59是一种嗜肝神经毒株,已知可诱导一系列应激途径,导致严重的神经炎症和脱髓鞘。先前的研究已经探索了MHV-A59或RSA59调节宿主应激途径以发挥其致病作用的能力。氧化应激和炎症应激是神经炎症和脱髓鞘的关键,导致不同的病毒诱导的神经变性和相关的发病机制。各种研究已经将iNOS在氧化-炎症应激串扰中的作用联系起来。本研究旨在通过神经元Neuro2A和星形细胞瘤DBT细胞的还原论模型研究M-CoV-MHV-RSA59感染中氧化应激和炎症通路的串音。我们的研究结果表明,虽然Neuro2A细胞表现出ROS水平的持续增加,导致Nrf2及其下游抗氧化酶HMOX1和过氧化氢酶的上调,但星形细胞瘤DBT细胞表现出短暂的ROS峰值,初始Nrf2下调,抗氧化反应受损。此外,我们观察到关键应激反应的差异调节,如XBP-1和DJ-1,突出了不同的细胞对病毒感染的适应。炎症介质NF-κB和iNOS的激活模式进一步强调了不同的炎症反应,在Neuro2A细胞中持续上调,而DBT细胞则表现出延迟激活。这些发现表明,神经元细胞参与内在的抗氧化防御,而神经胶质细胞则表现出受损的反应。我们的研究为神经胶质Neuro2A和DBT细胞以及mhv诱导的神经炎症中的氧化和炎症串音提供了机制见解,为改善神经退行性脱髓鞘疾病(如多发性硬化症)提供了潜在的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Murine β-coronavirus MHV-RSA59 orchestrates the interplay of oxidative stress and inflammatory pathways in neuroinflammation
Mouse Hepatitis Virus MHV-A59/RSA59 is hepato-neurotropic strain known to induce an array of stress pathways leading to severe neuroinflammation and demyelination. Previous studies have explored the ability of MHV-A59 or RSA59 to modulate host stress pathways to exert its pathogenic effects. Oxidative and inflammatory stress are critical in neuroinflammation and demyelination, leading to different virus-induced neurodegeneration and associated pathogenesis. Various studies have linked the role of iNOS in the crosstalk of oxidative-inflammatory stress. This study aims to investigate the crosstalk of oxidative stress and inflammatory pathways in M-CoV-MHV-RSA59 infection in a reductionist model in neuronal Neuro2A and astrocytoma DBT cells. Our findings demonstrate that while Neuro2A cells exhibited a persistent increase in ROS levels, leading to upregulation of Nrf2 and its downstream antioxidant enzymes HMOX1 and Catalase, astrocytoma DBT cells displayed a transient ROS peak with an initial downregulation of Nrf2 and impaired antioxidant response. Additionally, we observed differential regulation of key stress responders, such as XBP-1 and DJ-1, highlighting distinct cellular adaptations to viral infection. The inflammatory mediators NF-κB and iNOS activation patterns further underscored the differential inflammatory response, with sustained upregulation in Neuro2A cells, whereas DBT cells showed delayed activation. These findings suggest that neuronal cells engage an intrinsic antioxidant defense, while glial cells exhibit a compromised response. Our study provides mechanistic insights into oxidative and inflammatory crosstalk in the neuro-glial Neuro2A and DBT cells and in MHV-induced neuroinflammation, offering potential therapeutic targets for ameliorating neurodegenerative demyelinating disorders such as multiple sclerosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Virology
医学-病毒学
CiteScore
6.00
自引率
0.00%
发文量
157
审稿时长
50 days
期刊介绍:
Launched in 1955, Virology is a broad and inclusive journal that welcomes submissions on all aspects of virology including plant, animal, microbial and human viruses. The journal publishes basic research as well as pre-clinical and clinical studies of vaccines, anti-viral drugs and their development, anti-viral therapies, and computational studies of virus infections. Any submission that is of broad interest to the community of virologists/vaccinologists and reporting scientifically accurate and valuable research will be considered for publication, including negative findings and multidisciplinary work.Virology is open to reviews, research manuscripts, short communication, registered reports as well as follow-up manuscripts.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


