去质子化草甘膦的单分子分解:使用离子迁移率质谱法识别循环与线性脱水产物
IF 1.7
3区 化学
Q3 PHYSICS, ATOMIC, MOLECULAR & CHEMICAL
引用次数: 0
摘要
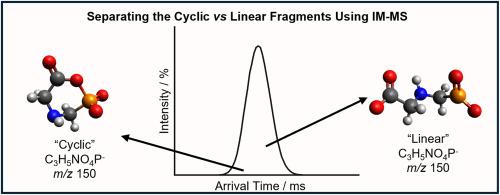
正如之前通过多级电喷雾电离质谱法观察到的那样,碰撞诱导解离(CID)脱质子草甘膦会导致几种分裂途径。这些过程包括脱羧和脱水,并根据断裂机制形成特征片段和异构体产物离子。本文研究了离子迁移率-质谱法(IM-MS)分离脱水草甘膦异构体的潜力。脱水草甘膦的两种异构体在单次循环离子迁移实验和多次循环实验中均未被分离。然而,使用特征片段,环状异构体似乎比线性异构体更稳定。本文首次观察到脱水草甘膦的CH4O3P−片段(m/z 95)。这被怀疑是在行波离子迁移(TWIMS)细胞中脱水草甘膦阴离子和本底水分子之间的离子分子反应的产物。脱水草甘膦离子水解生成膦酸甲酯阴离子和中性甘氨酸亚胺。13C标记和H/D交换实验支持了一个协调的机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Unimolecular decomposition of deprotonated glyphosate: Discerning cyclic versus linear dehydration products using ion mobility mass spectrometry
As observed previously by multistage electrospray ionisation mass spectrometry, collision induced dissociation (CID) of deprotonated glyphosate results in several fragmentation pathways. These include decarboxylation and dehydration, with the formation of characteristic fragments and isomeric product ions depending on the fragmentation mechanism. Herein, the potential of ion mobility – mass spectrometry (IM-MS) to separate the isomers of dehydrated glyphosate was investigated. Separation of the two isomers of dehydrated glyphosate was not observed with single-pass cyclic ion mobility experiments, nor by multi-pass experiments. However, using characteristic fragments the cyclic isomer appeared to be more stable the linear isomer. The CH4O3P− fragment (m/z 95) of dehydrated glyphosate was observed for the first time here. This is suspected to be the product of an ion molecule reaction between the dehydrated glyphosate anion and background water molecules within the travelling wave ion mobility (TWIMS) cell. Hydrolysis of the dehydrated glyphosate ion resulted in methyl phosphonate anion and neutral glycine imine formation. A concerted mechanism is supported by 13C labelling and H/D exchange experiments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.60
自引率
5.60%
发文量
145
审稿时长
71 days
期刊介绍:
The journal invites papers that advance the field of mass spectrometry by exploring fundamental aspects of ion processes using both the experimental and theoretical approaches, developing new instrumentation and experimental strategies for chemical analysis using mass spectrometry, developing new computational strategies for data interpretation and integration, reporting new applications of mass spectrometry and hyphenated techniques in biology, chemistry, geology, and physics.
Papers, in which standard mass spectrometry techniques are used for analysis will not be considered.
IJMS publishes full-length articles, short communications, reviews, and feature articles including young scientist features.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


