橙皮苷对酒精戒断综合征的神经保护作用:瑞士白化小鼠的临床前研究
Pharmacological Research - Modern Chinese Medicine
Pub Date : 2025-08-08
DOI:10.1016/j.prmcm.2025.100671
引用次数: 0
摘要
背景:橙皮苷(中文:苷,pí gān)是一种生物类黄酮,主要存在于柑橘类水果中,如橙子和橘子。几个世纪以来,它一直被用于中医(TCM),作为柑橘皮(Chénpí)和枳实(枳嘟嘟的,Zhǐshí)的关键活性成分,传统上用于治疗消化系统疾病,循环系统问题和呼吸系统疾病。这些草药在经典的中医配方中得到了证实,最近已被纳入中国流行病管理指南,突出了橙皮苷广泛的治疗潜力。现代研究已经证明橙皮苷具有很强的抗氧化、抗炎、神经保护和肝脏保护作用。由于这些特性,橙皮苷作为神经精神疾病如酒精戒断综合征(AWS)的天然治疗候选者获得了兴趣。因此,本研究旨在评估橙皮苷减轻小鼠AWS模型行为和生化改变的功效。方法雄性瑞士白化小鼠30只,每组6只,随机分为5组。4组小鼠连续21天给予含乙醇的改良液体日粮(MLD)诱导酒精依赖,对照组小鼠连续21天给予不含乙醇的改良液体日粮。乙醇退出后,小鼠以橙皮苷(50 mg/kg或100 mg/kg,口服)或地西泮(1 mg/kg,腹腔注射)作为参比标准48 h。在戒断后第6、24和48小时进行行为评估,采用高架加迷宫、空地测试(焦虑测试)、强迫游泳和悬尾测试(抑郁测试)、视压计(运动活动测试)和热板和尾巴浸入测试(伤害感受测试)。生化分析包括脑抗氧化酶,如超氧化物歧化酶(SOD)、过氧化氢酶(CAT)和谷胱甘肽(GSH),肝酶,如天冬氨酸转氨酶(AST)、丙氨酸转氨酶(ALT)和碱性磷酸酶(ALP)。结果与乙醇戒断组相比,橙皮苷在焦虑和抑郁样行为、运动活动、伤害反应、大脑抗氧化状态和肝酶水平方面具有统计学意义的改善。结论橙皮苷减轻了与AWS相关的行为、生化和组织病理学改变。虽然结果表明潜在的神经保护和肝脏保护作用可能涉及抗氧化机制,但这些发现是初步的。全面的药理学和机制研究是必要的,以确认其治疗相关性的AWS管理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
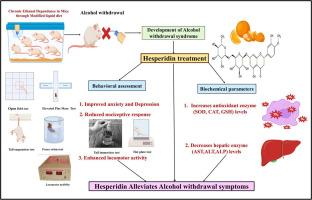
Neuroprotective potential of Hesperidin in Alcohol withdrawal syndrome: A preclinical study in Swiss albino mice
Background: Hesperidin (Chinese: 橙皮苷, Chéng pí gān) is a bioflavonoid primarily found in citrus fruits such as oranges and tangerines. It has been used for centuries in Traditional Chinese Medicine (TCM) as a key active component of Citri reticulatae Pericarpium (陈皮, Chénpí) and Aurantii fructus immaturus (枳实, Zhǐshí), which are traditionally employed to treat digestive disorders, circulatory problems, and respiratory ailments. These herbs are well-established in classical TCM formulations and have recently been included in Chinese guidelines for epidemic management, highlighting hesperidin’s broad therapeutic potential. Modern studies have demonstrated hesperidin’s strong antioxidant, anti-inflammatory, neuroprotective, and hepatoprotective effects. Owing to these properties, hesperidin has gained interest as a natural therapeutic candidate for neuropsychiatric conditions such as alcohol withdrawal syndrome (AWS). Accordingly, this study aimed to assess the efficacy of hesperidin in alleviating behavioral and biochemical alterations in a murine model of AWS.
Methods
Thirty male Swiss albino mice (n = 6 per group) were randomly divided into five groups. Four groups received a modified liquid diet (MLD) containing ethanol for 21 days to induce alcohol dependence, while the control group received an ethanol-free MLD. Upon ethanol withdrawal, mice were treated for 48 h with hesperidin (50 mg/kg or 100 mg/kg, orally) or diazepam (1 mg/kg, intraperitoneally), serving as the reference standard. Behavioral evaluations were conducted at 6th, 24th, and 48th h post-withdrawal using the elevated plus maze, open field test (for anxiety), forced swim and tail suspension tests (for depression), actophotometer (for locomotor activity), and hot plate and tail immersion tests (for nociception). Biochemical analyses included estimation of brain antioxidant enzymes such as, superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH), and liver enzymes like aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP).
Results
Hesperidin produced statistically significant improvements in anxiety- and depression-like behaviors, locomotor activity, nociceptive responses, brain antioxidant status, and hepatic enzyme levels compared to the ethanol withdrawal group.
Conclusion
Hesperidin alleviated behavioral, biochemical, and histopathological alterations associated with AWS in this study. Although the results indicate potential neuroprotective and hepatoprotective effects that may involve antioxidant mechanisms, these findings are preliminary. Comprehensive pharmacological and mechanistic studies are warranted to confirm its therapeutic relevance in AWS management.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


