可注射负载钠的丹参素缓释纳米颗粒水凝胶靶向巨噬细胞改善心肌微环境治疗心肌梗死
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
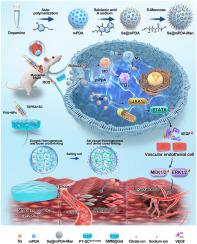
心肌梗死(MI)引起心肌细胞坏死、炎症、纤维化和心室重构,导致心力衰竭。为了解决这一问题,我们开发了一种智能心脏贴片SMM@Gel,它由一种活性氧(ROS)响应的PVA-TSPBA水凝胶基质组成,通过溶剂交换和盐化技术增强,装载甘露糖功能化、丹神素钠负载的中空介孔聚多巴胺纳米颗粒(Sa@mPDA-Man)。该设计实现了药物的持续释放和活性氧清除。体外研究表明,SMM@Gel促进内皮管形成(24±3个节点,对照组为6±2个)和M2巨噬细胞极化(CD206+细胞),同时减少炎症(iNOS下调)。在体内,心肌梗死大鼠的实验显示SMM@Gel保留了左心室射血分数(LVEF: 52.3%±4.1%,生理盐水组38.5%±3.2%),心室尺寸(EDV/ESV)正常化,壁厚增强。组织学分析显示梗死面积减小(18.7%±2.5% vs. 32.1%±3.8%),炎症减轻,新生血管改善。RNA-seq鉴定了与血管生成、炎症消退和细胞外基质重塑相关的途径。这些发现突出了SMM@Gel作为心肌梗死双作用疗法的潜力,结合ROS清除、抗炎作用和促进血管生成,以减轻心肌梗死后重构和保持心功能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Injectable sustained-release danshensu sodium-loaded nanoparticle hydrogel targets macrophages to improve myocardial microenvironment for myocardial infarction treatment
Myocardial infarction (MI) induces cardiomyocyte necrosis, inflammation, fibrosis, and ventricular remodeling, leading to heart failure. To address this, we developed an intelligent cardiac patch, SMM@Gel, composed of a reactive oxygen species (ROS)-responsive PVA-TSPBA hydrogel matrix reinforced via solvent exchange and salting-out technology, loaded with mannose-functionalized, danshensu sodium-loaded hollow mesoporous polydopamine nanoparticles (Sa@mPDA-Man). This design makes sustained drug release and ROS scavenging come true. In vitro, studies demonstrated SMM@Gel promoted endothelial tube formation (24 ± 3 nodes vs. 6 ± 2 in controls) and M2 macrophage polarization (CD206+ cells) while reducing inflammation (iNOS downregulation). In vivo, experiments in MI rats revealed SMM@Gel preserved left ventricular ejection fraction (LVEF: 52.3 % ± 4.1 % vs. 38.5 % ± 3.2 % in the saline group), normalized ventricular dimensions (EDV/ESV) and enhanced wall thickness. Histological analysis showed reduced infarct size (18.7 % ± 2.5 % vs. 32.1 % ± 3.8 %), decreased inflammation, and improved neovascularization. RNA-seq identified pathways linked to angiogenesis, inflammation resolution, and extracellular matrix remodeling. These findings highlight SMM@Gel's potential as a dual-action therapy for MI, combining ROS scavenging, anti-inflammatory effects, and angiogenic promotion to mitigate post-MI remodeling and preserve cardiac function.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


