基于MIL-100(Fe)的协同递送平台通过cGAS-STING途径作为结直肠癌级联增效化疗和免疫治疗药物
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
结直肠癌(CRC)仍然是全球主要的健康负担,是第三大最常诊断的恶性肿瘤,也是全球癌症相关死亡的第二大原因。虽然联合化疗和免疫激动剂具有通过多途径调节克服肿瘤异质性的潜力,但其治疗效果仍然受到脱靶药物分布和免疫抑制肿瘤微环境(TME)的限制。为了解决这个问题,我们开发了一种肿瘤靶向的化学免疫治疗平台,通过将伊立替康(CPT-11)和瑞喹莫特(R848)包被在透明质酸(HA)包被的MIL-100(Fe)金属有机框架(CRMH NPs)中,实现cd44介导的递送和协同抗肿瘤反应。该系统能够在酸性TME中控制药物释放,促进免疫原性细胞死亡(ICD),诱导cGAS-STING通路并激活免疫细胞。在体外,CRMH NPs诱导显著的ICD,通过释放损伤相关分子模式(DAMPs),如高迁移率组框1 (HMGB1)和钙网蛋白(CRT),以及成熟的树突状细胞(dc), TLR7激动剂触发,增强免疫应答。激活cGAS-STING通路,有效诱导靶细胞产生I型干扰素(IFN)和促炎细胞因子,增强T细胞的活化和募集。在体内,CRMH NPs显著降低CT26小鼠模型的肿瘤生长,激活先天免疫信号,增加免疫细胞浸润,与游离药物和其他纳米颗粒制剂相比,显示出优越的抗肿瘤作用。此外,CRMH NPs上调了肿瘤细胞上PD-L1的表达,提示其与免疫检查点阻断疗法有潜在的协同作用。CRMH NPs联合抗pd - l1治疗增强了抗肿瘤免疫,特别是在远处肿瘤中,突出了克服免疫逃避和实现持久肿瘤消退的有希望的方法。这些发现支持CRMH NPs作为CRC化疗免疫治疗的通用和有效平台。意义声明:结直肠癌(CRC)仍然是一个主要的全球健康挑战,由于肿瘤异质性和免疫抑制,治疗效果有限。本研究介绍了一种新的肿瘤靶向纳米颗粒平台,CRMH NPs,它使用透明质酸包被的MIL-100(Fe)金属有机框架,共同递送化疗药物伊立替康(CPT-11)和免疫激动剂雷昔莫特(R848)。CRMH NPs增强药物传递,诱导免疫原性细胞死亡,激活cGAS-STING通路,协同化疗和免疫治疗。该平台在临床前模型中展示了优越的肿瘤抑制和免疫激活,克服了当前治疗方法的关键局限性,如脱靶效应和免疫逃避。它联合抗pd - l1治疗进一步改善了预后,为持久的CRC治疗提供了一个有希望的策略。这项工作推进了纳米医学和免疫肿瘤学,为癌症治疗提供了一种变革性的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
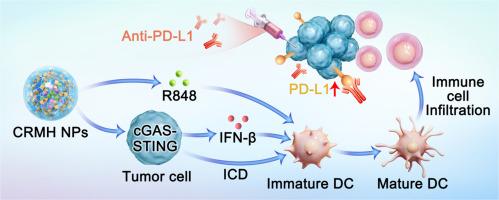
MIL-100(Fe)-based Co-delivery platform as cascade synergistic chemotherapy and immunotherapy agents for colorectal cancer via the cGAS-STING pathway
Colorectal cancer (CRC) remains a major global health burden as the third most commonly diagnosed malignancy and the second leading cause of cancer-related mortality worldwide. While combination chemotherapy and immune agonists hold potential to overcome tumor heterogeneity through multi-pathway modulation, their therapeutic efficacy remains limited by off-target drug distribution and immunosuppressive tumor microenvironment (TME). To address this, we developed a tumor-targeted chemo-immunotherapy platform by encapsulating irinotecan (CPT-11) and resiquimod (R848) in hyaluronic acid (HA) coated MIL-100(Fe) metal-organic frameworks (CRMH NPs), enabling CD44-mediated delivery and synergistic anti-tumor responses. The system enabled controlled drug release in the acidic TME, promoting immunogenic cell death (ICD), inducing the cGAS-STING pathway and activating immune cells. In vitro, CRMH NPs induced significant ICD, as evidenced by the release of damage-associated molecular patterns (DAMPs) like high mobility group box 1 (HMGB1) and calreticulin (CRT), and matured dendritic cells (DCs), which triggered by TLR7 agonist, enhancing immune responses. It also activated the cGAS-STING pathway, which could efficiently induce the targeted cells to produce type I interferon (IFN) and proinflammatory cytokines, thereby enhancing T cell activation and recruitment. In vivo, CRMH NPs significantly reduced tumor growth in CT26 mouse models, activated innate immune signaling, and increased immune cell infiltration, demonstrating superior anti-tumor effects compared to free drugs and other nanoparticle formulations. Furthermore, CRMH NPs upregulated PD-L1 expression on tumor cells, suggesting potential synergy with immune checkpoint blockade therapy. Combining CRMH NPs with anti-PD-L1 therapy enhanced anti-tumor immunity, particularly in distant tumors, highlighting a promising approach to overcoming immune evasion and achieving durable tumor regression. These findings supported CRMH NPs as a versatile and effective platform for chemo-immunotherapy in CRC.
Statement of Significance
Colorectal cancer (CRC) remains a major global health challenge with limited treatment efficacy due to tumor heterogeneity and immunosuppression. This study introduces a novel tumor-targeted nanoparticle platform, CRMH NPs, which co-deliver the chemotherapeutic drug irinotecan (CPT-11) and the immune agonist resiquimod (R848) using hyaluronic acid-coated MIL-100(Fe) metal-organic frameworks. CRMH NPs enhance drug delivery, induce immunogenic cell death, and activate the cGAS-STING pathway, synergizing chemotherapy and immunotherapy. Demonstrating superior tumor suppression and immune activation in preclinical models, this platform overcomes key limitations of current therapies, such as off-target effects and immune evasion. Its combination with anti-PD-L1 therapy further improves outcomes, offering a promising strategy for durable CRC treatment. This work advances nanomedicine and immuno-oncology, providing a transformative approach for cancer therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


