异茶碱B通过调节mavs介导的线粒体稳态减轻糖尿病诱导的心肌损伤。
IF 4.9
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
糖尿病性心肌病(DCM)是糖尿病死亡的主要原因,缺乏有效的治疗方法。本研究探讨了天然化合物异茶碱B (HET-B)在DCM中的心脏保护作用及其机制。使用链脲佐菌素诱导的DCM小鼠和高糖(HG)处理的H9C2/新生儿心肌细胞,我们评估了心功能、线粒体稳态和凋亡。HET-B显著改善心功能,表现为射血分数(EF)和分数缩短(FS)的增加。它还减少了心肌细胞凋亡(体内/体外),并改善了hg诱导的线粒体损伤,其特征是功能障碍、碎片化和过量活性氧(ROS)的产生。HET-B增强了线粒体融合蛋白OPA1的表达,减少了Bax/Bcl2、细胞色素C (Cyt C)的释放和caspase-3的切割。分子对接和细胞热移分析发现线粒体抗病毒信号蛋白(MAVS)是HET-B的潜在靶标。HET-B在体外和体内均可逆转HG/DCM诱导的MAVS下调。重要的是,通过siRNA敲低MAVS可消除HET-B对hg诱导的细胞凋亡和线粒体损伤的保护作用。此外,HET-B恢复hg损伤的自噬通量,减少自噬酶体的积累并使LC3-II正常化。总的来说,HET-B是一种天然化合物,通过靶向MAVS来减轻糖尿病心肌损伤,从而增强自噬,保持线粒体稳态,抑制细胞凋亡,使其成为一种有希望的DCM治疗候选药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
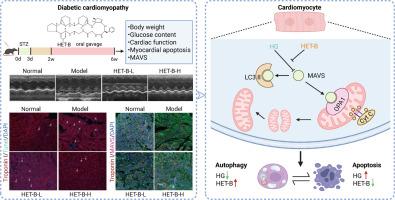
Heterophyllin B alleviates diabetes-induced myocardial injury by regulating MAVS-mediated mitochondrial homeostasis
Diabetic cardiomyopathy (DCM), a major cause of diabetic mortality, lacks effective therapies. This study investigated the cardioprotective role of Heterophyllin B (HET-B), a natural compound and its underlying mechanisms in DCM. Using streptozotocin-induced DCM mice and high glucose (HG)-treated H9C2/neonatal cardiomyocytes, we assessed cardiac function, mitochondrial homeostasis, and apoptosis. HET-B significantly improved cardiac function, indicated by increased ejection fraction (EF) and fractional shortening (FS). It also reduced cardiomyocyte apoptosis (in vivo/vitro), and ameliorated HG-induced mitochondrial damage, characterized by dysfunction, fragmentation, and excessive reactive oxygen species (ROS) production. HET-B enhanced mitochondrial fusion protein OPA1 expression and reduced Bax/Bcl2, cytochrome C (Cyt C) release and caspase-3 cleavage. Molecular docking and cellular thermal shift assays identified mitochondrial antiviral-signaling protein (MAVS) as a potential target of HET-B. HET-B reversed MAVS downregulation induced by HG/DCM in vitro and in vivo. Importantly, MAVS knockdown via siRNA abolished HET-B's protection against HG-induced apoptosis and mitochondrial damage. Furthermore, HET-B restored HG-impaired autophagic flux, reducing autolysosome accumulation and normalizing LC3-II. Collectively, the natural compound HET-B alleviates diabetic myocardial injury by targeting MAVS to enhance autophagy, maintain mitochondrial homeostasis, and inhibit cardiomyocyte apoptosis, positioning it as a promising therapeutic candidate for DCM.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Nutritional Biochemistry
医学-生化与分子生物学
CiteScore
9.50
自引率
3.60%
发文量
237
审稿时长
68 days
期刊介绍:
Devoted to advancements in nutritional sciences, The Journal of Nutritional Biochemistry presents experimental nutrition research as it relates to: biochemistry, molecular biology, toxicology, or physiology.
Rigorous reviews by an international editorial board of distinguished scientists ensure publication of the most current and key research being conducted in nutrition at the cellular, animal and human level. In addition to its monthly features of critical reviews and research articles, The Journal of Nutritional Biochemistry also periodically publishes emerging issues, experimental methods, and other types of articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


