四芳基偶氮双吡咯的合成与性质。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
偶氮双吡咯框架,它融合了偶氮(-N -N -)部分和吡咯杂环的令人印象深刻的电子能力,其化学性质明显不发达。本文介绍了一系列由四个芳基取代的偶氮双吡咯的合成和表征。当使用硝基丁酮作为起始原料时,吡咯基的β-位置上芳基的空间体积控制了偶氮双吡咯生成与偶氮双吡咯生成的竞争程度。讨论了吡啶基α-位置上芳基对构象稳定性的影响,以及由此产生的对光物理性质的控制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
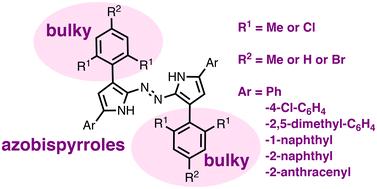
Synthesis and properties of tetra-aryl azobispyrroles
The chemistry of the azobispyrrole framework, which melds the impressive electronic capabilities of the azo (–NN–) moiety and the pyrrole heterocycle, is significantly under-developed. Herein, the synthesis and characterisation of a series of azobispyrroles substituted with four aryl groups are presented. The steric bulk of aryl groups at the β-positions of the pyrrolic building blocks controls the degree to which azobispyrrole formation competes with aza-dipyrrin formation when using nitrobutanones as starting materials. The ability of aryl groups at the α-positions of the pyrrolic building blocks to influence conformational stability is discussed, as is the consequent control of photophysical properties.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


