通过分子内c2酰胺化酰胺系c3 -亚砜吡啶合成吲哚-融合苯并噻吩酮。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
一种无过渡金属,碘介导的策略已经开发用于合成具有生物意义的吲哚融合苯并噻吩类酮。该方法首先对吲哚进行亲电性C3碘化,然后对易于获得的酰胺系C3-磺酰吲哚进行分子内C2酰胺化,以获得收率高的吲哚融合苯并噻唑类药物。该协议具有广泛的基板兼容性、高功能组容忍度和可扩展性。此外,通过转化为相应的亚砜和砜,证明了所得到的吲哚-融合苯并噻吩类酮的合成通用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
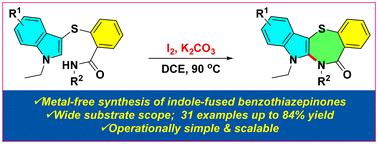
Iodine-mediated synthesis of indole-fused benzothiazepinones through intramolecular C2-amidation of amide-tethered C3-sulfenylindoles
A transition-metal-free, iodine-mediated strategy has been developed for the synthesis of biologically significant indole-fused benzothiazepinones. This method involves an initial electrophilic C3 iodination of indole, followed by intramolecular C2 amidation of readily accessible amide-tethered C3-sulfenylindoles to afford indole-fused benzothiazepinones in good yields. The protocol exhibits broad substrate compatibility, high functional group tolerance, and scalability. Additionally, the synthetic versatility of the resulting indole-fused benzothiazepinones was demonstrated through their transformation into the corresponding sulfoxides and sulfones.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


