cgrp相关神经肽肾上腺髓质素2促进组织保护性ILC2反应并限制肠道炎症
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
神经免疫回路调节屏障表面的先天免疫和适应性免疫。然而,这些回路对促炎反应和组织保护反应的不同影响仍然不清楚。我们证明了肠道神经元产生降钙素基因相关肽相关肾上腺髓质素2 (ADM2),并确定了ADM2途径在促进2组先天淋巴样细胞(ILC2s)肠道组织保护功能中的先前未被认识的作用。ADM2受体亚基的基因组或ILC2内在缺失导致组织保护性ILC2反应显著减少,双调节蛋白(AREG)产生缺陷,肠道损伤和炎症易感性增加。相反,治疗性递送重组ADM2可诱导组织保护性AREG+ ILC2s,并限制肠道炎症。在炎症性肠病患者中,编码人类ADM2受体(CALCRL和RAMP3)的基因表达发生改变,并与ILC2s中AREG表达降低相关。综上所述,这些发现表明ADM2-ADM2受体通路可以在肠道损伤和炎症的情况下促进ILC2s的组织保护功能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
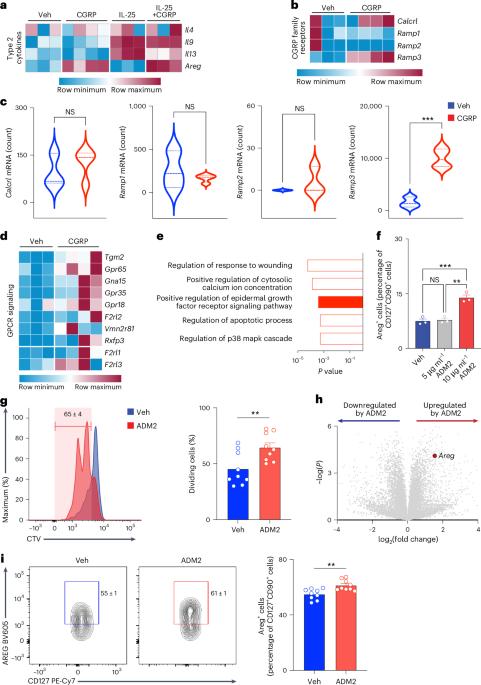
CGRP-related neuropeptide adrenomedullin 2 promotes tissue-protective ILC2 responses and limits intestinal inflammation
Neuro–immune circuits regulate innate and adaptive immunity at barrier surfaces. However, the differential impact of these circuits on proinflammatory versus tissue-protective responses remains poorly defined. We demonstrate that enteric neurons produce calcitonin gene-related peptide-related adrenomedullin 2 (ADM2) and identify a previously unrecognized role for the ADM2 pathway in promoting intestinal tissue-protective functions of group 2 innate lymphoid cells (ILC2s). Genomic or ILC2-intrinsic deletion of ADM2 receptor subunits resulted in a significant reduction in tissue-protective ILC2 responses, defective amphiregulin (AREG) production and increased susceptibility to intestinal damage and inflammation. Conversely, therapeutic delivery of recombinant ADM2 elicited tissue-protective AREG+ ILC2s and limited intestinal inflammation. Expression of genes encoding human ADM2 receptor (CALCRL and RAMP3) was altered in participants with inflammatory bowel diseases and associated with reduced expression of AREG in ILC2s. Collectively, these findings identify that the ADM2–ADM2 receptor pathway can promote tissue-protective functions of ILC2s in the context of intestinal damage and inflammation. Artis and colleagues show that enteric neurons produce CGRP-related ADM2 to promote intestinal tissue-protective functions in ILC2s.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


