双载体rAAVrh8基因治疗GM2神经节脂质病:1/2期试验
IF 50
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
双重rAAVrh8-HEXA和rAAVrh8-HEXB载体可恢复动物模型双丘脑(BiT)注射后中枢神经系统hexosaminidase (Hex)酶活性,降低脑脊液GM2水平,挽救GM2神经节脂质病、Tay-Sachs病和Sandhoff病的表型后果。在一项n = 2的扩大准入试验之后,我们启动了一项1/2期、单剂量、剂量递增的联合BiT、大池内和鞘内输注治疗Tay-Sachs和Sandhoff病儿童(6名婴儿,3名青少年)。在四个队列中,比特币注射量和载体剂量增加了一倍,最低剂量与早期扩大准入试验相匹配。脑脊液HexA酶活性、血清总HexA活性和GM2水平显示出剂量依赖性的疾病生化校正。血清Hex活性超过40 nmol h - 1 ml - 1,是正常下限的两倍,神经影像学显示纤维束增加。校正在12周时最大,但在给药后24周时下降。婴儿患者在3-3.5岁前经历了总体临床稳定和无误吸的长期口服喂养。癫痫发作时间较晚,频率较低,严重程度较轻,对抗惊厥药物反应较好。不良事件在婴儿患者中很少见,但在青少年患者中观察到肌张力障碍的恶化,他们被排除在正在进行的研究中。ClinicalTrials.gov注册号:NCT04669535和NCT06614569。本文章由计算机程序翻译,如有差异,请以英文原文为准。
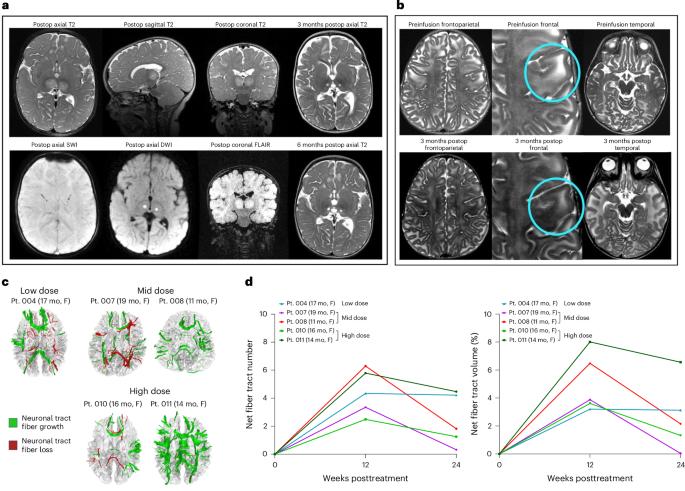
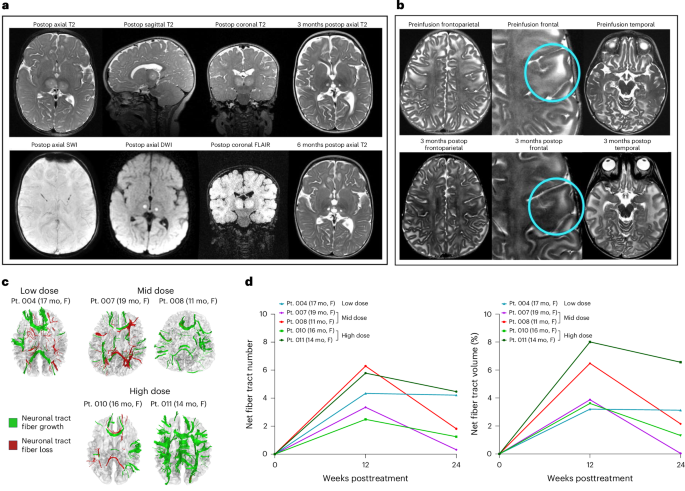
Dual-vector rAAVrh8 gene therapy for GM2 gangliosidosis: a phase 1/2 trial
The dual rAAVrh8-HEXA and rAAVrh8-HEXB vector can restore central nervous system hexosaminidase (Hex) enzyme activity, decrease GM2 levels in cerebrospinal fluid and rescue phenotypic consequences of GM2 gangliosidosis, Tay–Sachs and Sandhoff diseases in animal models following simultaneous bi-thalamic (BiT) injections. Following up on an n = 2 expanded access trial, we initiated a phase 1/2, single-dose, dose-escalation of combined BiT, intra-cisterna magna and intrathecal infusion in children with Tay–Sachs and Sandhoff diseases (six infantile, three juvenile). The BiT injection volume and vector dose were doubled between four cohorts, with the lowest dose matching the earlier expanded access trial. Cerebrospinal fluid HexA enzyme activity, serum total Hex activity and GM2 levels showed a dose-dependent biochemical correction of the disease. Serum Hex activity surpassed 40 nmol h−1 ml−1, two times the lower limit of normal, and neuroimaging demonstrated increased fiber tracts. Correction was greatest at 12 weeks, but in decline by 24 weeks postdosing. Infantile patients experienced global clinical stabilization and prolonged oral feeding without aspiration until 3–3.5 years. Seizures had a later onset, were less frequent, less severe and more responsive to anti-convulsant medication. Adverse events were rare in infantile patients, but worsening dystonia was observed in juvenile patients, who were excluded from ongoing enrollment. ClinicalTrials.gov registration: NCT04669535 and NCT06614569 . A phase 1/2 trial of dual-vector rAAVrh8 gene therapy for GM2 gangliosidosis, administered by bilateral intrathalamic, cisterna magna and intrathecal delivery found a dose-dependent biochemical correction of the disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


