通过电化学阻抗数据的图形化处理,探索CoCrFeNiMo0.4高熵合金在含h2o2电解质中形成钝化膜的保护性能
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
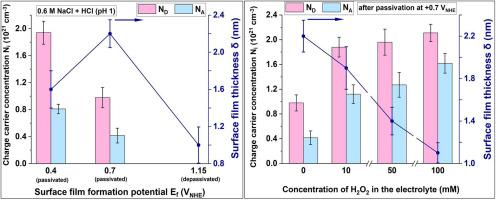
研究了CoCrFeNiMo0.4高熵合金在0.6M NaCl + HCl (pH 1)和H2O2(10、50、100 mM)存在下钝化和钝化状态下的电化学行为。通过极化测量、Mott-Schottky分析和电化学阻抗谱对表面膜的性质进行了研究,并利用图形化方法对结果进行了强化处理。测定了钝化层的定量参数,即氧化物/电解质界面处的电阻率和载流子浓度。同时,利用复电容表示法计算了薄膜厚度。无论H2O2浓度如何,合金都发生自发钝化,但随着氧化剂含量的增加,钝化状态下的阳极溶解加剧。随着背景溶液中地层电位的升高,钝化层变厚,导电性降低,但随着H2O2浓度的增加,钝化层变薄,缺陷增多。然而,无论H2O2含量如何,界面电阻率保持在106 Ω∙cm的水平。由此可见,氧化剂对钝化层保护能力的不利影响较弱,说明了钝化状态的高稳定性和有效性。在钝化电位处,保护能力下降,界面电阻率下降,薄膜变薄。在H2O2存在的情况下,这种下降更为明显。薄膜性质的变化与极化测量结果一致。此外,与316L钢相比,该合金表现出更好的钝化层性能,表明其具有更高的耐腐蚀性。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Exploring protective properties of passive films formed upon CoCrFeNiMo0.4 high-entropy alloy in H2O2-containing electrolytes via graphical processing of electrochemical impedance data
The electrochemical behavior of CoCrFeNiMo0.4 high-entropy alloy both in the passive state and at depassivation in 0.6 M NaCl + HCl (pH 1) and in the presence of H2O2 (10, 50, or 100 mM) was studied. The surface film properties were investigated by means of polarization measurements, Mott-Schottky analysis, and electrochemical impedance spectroscopy utilizing graphical methods for enhanced processing of the results. The quantitative parameters of the passive layer, namely its resistivity at the oxide/electrolyte interface and the charge carrier concentrations, were determined. Also, the film thickness was calculated using the complex capacitance representation. The alloy underwent spontaneous passivation regardless of the H2O2 concentration, but the anodic dissolution in the depassivated state intensified as the oxidizer content increased. The passive layer became thicker and less conductive with rising formation potential in the background solution, but became thinner and more defective as the H2O2 concentration increased. Nevertheless, the interfacial resistivity remained at the level of 106 Ω∙cm, irrespective of the H2O2 content. Thus, the detrimental effect of the oxidizer on the protective ability of the passive layer was weak, indicating the high stability and effectiveness of the passive state. Deterioration in the protective ability occurred at the depassivation potential, as evidenced by the decrease in interfacial resistivity and thinning of the film. The decline was more pronounced in the presence of H2O2. The variations of the film properties were consistent with the polarization measurements. Additionally, the alloy exhibited better passive layer properties compared to the 316L steel, indicating its higher corrosion resistance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


