人SLC26A7底物识别机制的结构基础
IF 15.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
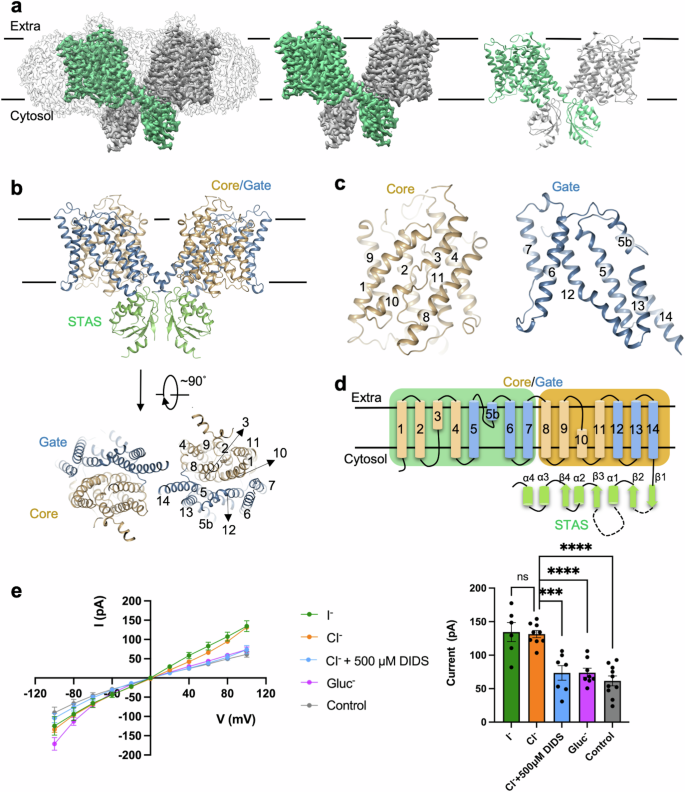
溶质载体家族26 (SLC26)主要介导多种阴离子的跨膜转运,包括氯离子等卤化物离子、碳酸氢盐、草酸盐、硫酸盐等。许多严重的人类遗传性疾病都与SLC26蛋白突变有关。在这里,我们报道了人类SLC26A7在载脂蛋白和碘化物结合状态下的低温电镜结构。我们确定了SLC26A7中卤化物离子的非规范结合位点。分子动力学模拟和电生理分析证实了参与碘化物和氯化物配位的关键残基的功能重要性。总之,我们的发现标志着深入了解SLC26家族蛋白转运机制迈出了一步。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Structural basis for substrate recognition mechanism of human SLC26A7
Solute carrier family 26 (SLC26) mainly mediates transmembrane transport of various anion ions, including chloride and other halide ions, bicarbonate, oxalate, and sulfate. Many severe hereditary human diseases are correlated with SLC26 protein mutations. Here we report cryo-EM structures of human SLC26A7 in apo and iodide binding states. We identify non-canonical binding site for halide ions in SLC26A7. Molecular dynamics simulation and electrophysiological assay confirm the functional importance of key residues involved in iodide and chloride coordination. Together, our discovery marks a step towards an in-depth understanding of SLC26 family protein transport mechanisms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Communications
Biological Science Disciplines-
CiteScore
24.90
自引率
2.40%
发文量
6928
审稿时长
3.7 months
期刊介绍:
Nature Communications, an open-access journal, publishes high-quality research spanning all areas of the natural sciences. Papers featured in the journal showcase significant advances relevant to specialists in each respective field. With a 2-year impact factor of 16.6 (2022) and a median time of 8 days from submission to the first editorial decision, Nature Communications is committed to rapid dissemination of research findings. As a multidisciplinary journal, it welcomes contributions from biological, health, physical, chemical, Earth, social, mathematical, applied, and engineering sciences, aiming to highlight important breakthroughs within each domain.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


