通过Srb选择性摄取途径将胆固醇转运到卵巢支持三瘤蟹(Portunus trituberculatus)中星介导的甾体生成。
IF 4.9
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
背景:胆固醇是类固醇生成的必需物质,在卵巢发育中起关键作用。在甲壳类动物中,清道夫受体B类成员(Srb)和甾体生成急性调节蛋白(Star)可能分别是胆固醇转运和甾体生成的关键蛋白。目的:通过体内和体外实验,探讨胆固醇在雌梭子蟹甾体生成和卵巢发育中的作用。方法:对照饲粮(Ctrl,不添加胆固醇)和胆固醇饲粮(CH)分别饲喂游泳蟹,分别在第1期(30 d)和第2期卵巢发育(60 d)中取样。通过敲低和过表达实验评估Srb和Star功能。采用生化指标、组织学、免疫荧光、基因表达和Western blot评价胆固醇转运、甾体生成和卵巢发育。利用HEK293T细胞进行免疫荧光和共免疫沉淀研究机制。结果:补充胆固醇显著增加类固醇激素和卵黄蛋白原(VTG)浓度,以及胆固醇转运、类固醇生成和VTG / VTG mRNA和蛋白表达水平。干扰和过表达Srb后,结果表明Srb通过调节胆固醇外排基因abcg1维持胆固醇稳态,而不是通过代偿性调节ldl。在体外也发现了类似的模式,srb介导的胆固醇转运促进了类固醇激素的产生。此外,star敲低可减少甾体生成和vtg/ vtg表达,而star过表达可逆转这一过程。结论:研究结果验证了Srb在胆固醇转运和Star在类固醇形成中的作用,表明胆固醇补充可通过Srb选择性摄取途径促进胆固醇转运,支持Star介导的类固醇形成,从而促进梭子蟹卵巢发育。本文章由计算机程序翻译,如有差异,请以英文原文为准。
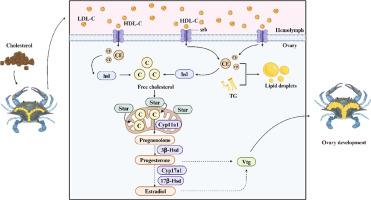
Transport of cholesterol to ovary via the Srb selective uptake pathway supports Star-mediated steroidogenesis in swimming crab (Portunus trituberculatus)
Cholesterol, essential for steroidogenesis, is crucial in ovarian development. In crustaceans, scavenger receptor class B member (Srb) and steroidogenic acute regulatory protein (Star) may be key proteins in cholesterol transport and steroidogenesis, respectively. This study aimed to explore cholesterol's role in steroidogenesis and ovarian development in female swimming crabs through in vivo and in vitro experiments. The control diet (Ctrl, without added cholesterol) and the cholesterol diet (CH) were fed swimming crabs and sampled first (30 d) and second ovarian development (60 d). Srb and Star functions were assessed via knockdown and overexpression experiments. Biochemical indices, histology, immunofluorescence, gene expression and Western blot were used to evaluate cholesterol transport, steroidogenesis and ovarian development. HEK293T cells were used for immunofluorescence and co-immunoprecipitation to explore mechanisms. Cholesterol supplementation significantly increased steroid hormone and vitellogenin (VTG) concentrations, as well as mRNA and protein expression levels of cholesterol transport, steroidogenesis, and vtg/Vtg. After interference and overexpression of Srb, the results indicated that Srb maintained cholesterol homeostasis by regulating cholesterol efflux gene abcg1, but not through compensatory regulation of ldlr. Similar patterns were found in vitro, Srb-mediated cholesterol transport promoted steroid hormone production. Moreover, star knockdown reduced steroidogenesis and vtg/Vtg expression, which was reversed by Star overexpression. The findings verified the roles of Srb in cholesterol transport and Star in steroidogenesis, and revealed that cholesterol supplementation promoted cholesterol transport via the Srb-selective uptake pathway, and supported Star-mediated steroidogenesis, thereby promoting ovarian development in swimming crab.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Nutritional Biochemistry
医学-生化与分子生物学
CiteScore
9.50
自引率
3.60%
发文量
237
审稿时长
68 days
期刊介绍:
Devoted to advancements in nutritional sciences, The Journal of Nutritional Biochemistry presents experimental nutrition research as it relates to: biochemistry, molecular biology, toxicology, or physiology.
Rigorous reviews by an international editorial board of distinguished scientists ensure publication of the most current and key research being conducted in nutrition at the cellular, animal and human level. In addition to its monthly features of critical reviews and research articles, The Journal of Nutritional Biochemistry also periodically publishes emerging issues, experimental methods, and other types of articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


