时空纳米生物矿化和促抗生素释放协同对抗多药耐药细菌
IF 22
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
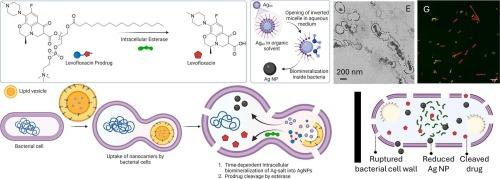
随着微生物耐药性的出现和普遍存在,细菌感染和相关疾病成为全球健康问题的主要原因。许多因素,包括阻碍制药业生产新抗生素的监管限制,使得必须开发一个平台,以对抗具有已知药物和可药物替代品的多重耐药细菌。在这里,我们提出了一种组合方法,利用银纳米颗粒的杀菌特性以及嵌入在脂质纳米颗粒中的左氧氟沙星前药。联合前药技术和生物矿化的时空协同效应能够在细菌细胞内实现靶向、可控的药物释放,提高生物利用度,克服耐药性,最大限度地减少全身毒性,同时利用原位银纳米颗粒形成来增强抗菌功效。我们的研究报告了细菌内部形成直径为~ 100±20 nm的均匀纳米颗粒,从而协同增强杀菌效果。革兰氏阳性枯草芽孢杆菌的体外研究对细菌细胞内脂质纳米颗粒内银离子的摄取和还原机制产生了重要的见解,并已推广到体内研究。多药耐药革兰氏阴性菌肺炎克雷伯菌的体外研究进一步巩固了这一概念。我们进一步利用高光谱成像、共聚焦显微镜和质谱分析显示,在细菌酯酶存在的情况下,纳米载体的细胞内摄取和前药的裂解具有时间依赖性。在啮齿动物模型中进行的体内研究也显示,与单个成分相比,纳米载体的杀菌性能显著增强,并证明了它们的生物相容性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Spatio-temporal nano-biomineralization and pro-antibiotic release for synergistically combatting multidrug-resistant bacteria
With the emergence and prevalence of drug resistance in microorganisms, bacterial infections and related diseases are major causes of global health concerns. A host of factors, including regulatory restrictions hindering the production of new antibiotics by the pharmaceutical industries, makes it essential to develop a platform for combatting multidrug-resistant bacteria with known drugs and druggable alternatives. Herein, we present a combinatorial approach that harnesses the bactericidal properties of silver nanoparticles along with a levofloxacin prodrug embedded within the lipid nanoparticle. The spatio-temporal synergistic effect of combined prodrug technology and biomineralization enables targeted, controlled drug release within bacterial cells, enhancing bioavailability, overcoming drug resistance, and minimizing systemic toxicity while leveraging in-situ silver nanoparticle formation for amplified antimicrobial efficacy. Our studies report the formation of homogeneous nanoparticles of ∼ 100 ± 20 nm in diameter within bacteria resulting in synergistic enhancement of bactericidal effect. The in-vitro studies with Gram-positive Bacillus subtilis resulted in important insight into the mechanism of uptake and reduction of silver ion incorporated within the lipid nanoparticles inside the bacterial cell, which have been extrapolated to in-vivo studies. This concept was further consolidated with in-vitro studies on multidrug-resistant Gram-negative bacteria Klebsiella pneumoniae. We further showed time-dependent intracellular uptake of the nanocarriers and cleavage of the prodrug in the presence of bacterial esterase enzyme using hyperspectral imaging, confocal microscopy, and mass spectrometry. In vivo studies carried out in a rodent model also revealed significant enhancement of the nanocarriers’ bactericidal properties compared to individual components and demonstrated their biocompatibility.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today
工程技术-材料科学:综合
CiteScore
36.30
自引率
1.20%
发文量
237
审稿时长
23 days
期刊介绍:
Materials Today is the leading journal in the Materials Today family, focusing on the latest and most impactful work in the materials science community. With a reputation for excellence in news and reviews, the journal has now expanded its coverage to include original research and aims to be at the forefront of the field.
We welcome comprehensive articles, short communications, and review articles from established leaders in the rapidly evolving fields of materials science and related disciplines. We strive to provide authors with rigorous peer review, fast publication, and maximum exposure for their work. While we only accept the most significant manuscripts, our speedy evaluation process ensures that there are no unnecessary publication delays.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


