用于向线虫递送dsRNA的基于蛋白质的球形纳米颗粒- RNA沉默的平台技术
IF 22
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
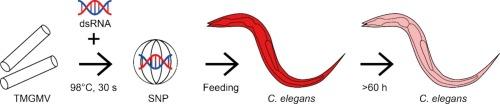
植物寄生线虫是广泛存在的农业害虫,对根系造成严重损害,造成重大作物损失。用杀线虫剂进行化学防治是传统的害虫管理策略,但这对有益物种和人类健康构成威胁。此外,不加选择地使用会导致抗性害虫种群的出现。植物寄生线虫也可以受到RNA干扰(RNAi)的控制,RNAi是一种真核生物防御侵入性核酸的机制,由双链RNA (dsRNA)触发,并导致相应mRNA的特异性切割或翻译抑制。在实验室条件下,已有超过75个植物寄生线虫的基因被RNAi靶向,但RNAi在该领域的应用受到诸如细胞摄取效率低下和RNA降解等递送障碍的限制。由于游离RNA在土壤中不稳定,因此在针对土壤线虫时,后者尤其重要。因此,我们将dsRNA封装在由烟草温和绿色花叶病毒(TMGMV)外壳蛋白热退火形成的蛋白球状纳米颗粒(snp)中。我们通过电荷中和和Mg2+在pH <下的dsRNA缩合来优化dsRNA的SNPs负载;3.0,允许我们封装高达0.2毫克的dsRNA每1.0毫克的snp,直径100 - 200nm。这比未优化的dsRNA- snp配方(即没有电荷中和和冷凝的dsRNA封装)提高了10倍。一种表达mCherry的转基因秀丽隐杆线虫系被用作模型,以证实dsRNA在摄入含有dsRNA的snp后仍保持功能并触发RNAi。沉默效果持续约180 h, mCherry荧光降低76.2±13.6%。我们证实负载dsrna的SNPs在通过土壤柱时保持其沉默活性,这表明使用SNPs基于rnai的植物寄生线虫控制应该是可能的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Protein-based spherical nanoparticles for dsRNA delivery to nematodes – A platform technology for RNA silencing
Phytoparasitic nematodes are widespread agricultural pests that cause severe damage to roots, resulting in significant crop losses. Chemical control with nematicides is the conventional pest management strategy, but this is a threat to beneficial species and human health. Furthermore, indiscriminate use leads to the emergence of resistant pest populations. Phytoparasitic nematodes can also be controlled by RNA interference (RNAi), a eukaryote defense mechanism against invasive nucleic acids that is triggered by double stranded RNA (dsRNA) and causes the specific cleavage or translational repression of the corresponding mRNA. More than 75 genes in phytoparasitic nematodes have been targeted by RNAi under laboratory conditions, but the application of RNAi in the field is limited by delivery barriers such as inefficient cellular uptake and RNA degradation. The latter is particularly important when targeting soil-dwelling nematodes because free RNA is not stable in soil. We therefore encapsulated dsRNA in proteinaceous spherical nanoparticles (SNPs) formed by the thermal annealing of coat proteins from tobacco mild green mosaic virus (TMGMV). We optimized loading of dsRNA into SNPs by charge neutralization and condensation of dsRNA with Mg2+ at pH < 3.0, allowing us to encapsulate up to 0.2 mg dsRNA per 1.0 mg of SNPs, 100–200 nm in diameter. This was a 10-fold improvement over the non-optimized dsRNA-SNP formulation (i.e. encapsulation of dsRNA without charge neutralization and condensation). A transgenic Caenorhabditis elegans line constitutively expressing mCherry was used as a model to confirm that dsRNA remains functional and triggers RNAi following the ingestion of dsRNA-laden SNPs. The silencing effect lasted ∼180 h and reduced mCherry fluorescence by 76.2 ± 13.6 %. We confirmed that dsRNA-loaded SNPs retain their silencing activity when passed through a soil column, indicating that the RNAi-based control of phytoparasitic nematodes using SNPs should be possible in the field.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today
工程技术-材料科学:综合
CiteScore
36.30
自引率
1.20%
发文量
237
审稿时长
23 days
期刊介绍:
Materials Today is the leading journal in the Materials Today family, focusing on the latest and most impactful work in the materials science community. With a reputation for excellence in news and reviews, the journal has now expanded its coverage to include original research and aims to be at the forefront of the field.
We welcome comprehensive articles, short communications, and review articles from established leaders in the rapidly evolving fields of materials science and related disciplines. We strive to provide authors with rigorous peer review, fast publication, and maximum exposure for their work. While we only accept the most significant manuscripts, our speedy evaluation process ensures that there are no unnecessary publication delays.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


