辅料中亚硝酸盐的检测。工业最佳实践
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
硝亚胺风险评估与控制是药品开发和产品评价的重要组成部分。辅料中的亚硝酸盐对原料药、原料药杂质或其他工艺相关胺中存在的易损胺构成风险,因为它们可以亚硝化形成n -亚硝胺。对赋形剂中的亚硝酸盐进行检测和定量是制药业的一项重要工作,可为亚硝胺风险评估和相关风险缓解战略提供信息。建立了一个行业联盟和拉萨亚硝酸盐数据库,以合作应对这一挑战,分享知识,减少检测负担。本文展示了该联盟对辅料中亚硝酸盐定量分析技术的现有理解,包括IC与电导率或UV检测,Griess衍生化(随后进行HPLC-UV或MS/MS检测或IC后作为PCD), DAN衍生化(FL和MS检测)和环己基磺酸衍生化(GC-FID或GC-MS检测)。我们的目标是强调各种最佳实践,并详细介绍它们的技术原理、性能特征和样品制备。利用数据库中的亚硝酸盐结果突出了可以实现的亚硝酸盐loq范围,以及使用每种分析技术的优点和缺点的知识。本出版物的目的是方便选择适当的分析方法时,考虑亚硝酸盐的辅料测定。本文章由计算机程序翻译,如有差异,请以英文原文为准。
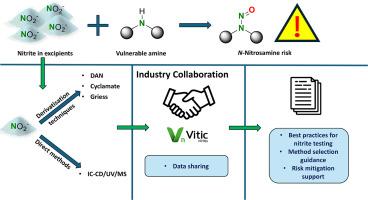
Nitrite testing in excipients – Industry best practices
N![]() Nitrosamine risk assessment and control are essential components of pharmaceutical drug development and product evaluation. Nitrites present in excipients pose a risk to vulnerable amines that are present in APIs, API impurities or other process related amines, as they can be nitrosated to form N-nitrosamines. Detection and quantification of nitrite in excipients is an essential undertaking within the pharmaceutical industry to inform nitrosamine risk assessment and related risk mitigation strategies. An industry consortium and Lhasa Nitrites database was established to collaborate on this challenge, share knowledge, and reduce the testing burden. This article demonstrates the existing understanding of analytical techniques within this consortium for the quantification of nitrite in excipients incorporating IC with conductivity or UV detection, Griess derivatisation (with subsequent HPLC-UV or MS/MS detection or as PCD after IC), DAN derivatisation (with FL and MS detection) and cyclamate derivatisation (with GC-FID or GC–MS detection). We aim to highlight a variety of best practices as well as detailing their techniques principles, performance characteristics and sample preparation. Utilising the nitrite results in the database has highlighted a range in LOQs of nitrite that can be achieved, as well as knowledge of the advantages and disadvantages of using each analytical technique. This publication aims to facilitate the selection of an appropriate analytical method when considering nitrite in excipient determination.
Nitrosamine risk assessment and control are essential components of pharmaceutical drug development and product evaluation. Nitrites present in excipients pose a risk to vulnerable amines that are present in APIs, API impurities or other process related amines, as they can be nitrosated to form N-nitrosamines. Detection and quantification of nitrite in excipients is an essential undertaking within the pharmaceutical industry to inform nitrosamine risk assessment and related risk mitigation strategies. An industry consortium and Lhasa Nitrites database was established to collaborate on this challenge, share knowledge, and reduce the testing burden. This article demonstrates the existing understanding of analytical techniques within this consortium for the quantification of nitrite in excipients incorporating IC with conductivity or UV detection, Griess derivatisation (with subsequent HPLC-UV or MS/MS detection or as PCD after IC), DAN derivatisation (with FL and MS detection) and cyclamate derivatisation (with GC-FID or GC–MS detection). We aim to highlight a variety of best practices as well as detailing their techniques principles, performance characteristics and sample preparation. Utilising the nitrite results in the database has highlighted a range in LOQs of nitrite that can be achieved, as well as knowledge of the advantages and disadvantages of using each analytical technique. This publication aims to facilitate the selection of an appropriate analytical method when considering nitrite in excipient determination.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


