调节耐高温锂硫电池的电解质溶剂化结构和离子动力学
IF 22
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
传统电解质由于热稳定性不足、副反应加剧,极大地限制了锂硫电池在高温下的有效运行,电池失效机理和电解质设计原理一直是一个谜。在此,我们开发了一种变温多模态核磁共振(VT-mNMR)方法来阐明温度依赖的电解质溶剂化结构和离子动力学,从而制定了耐热双溶剂化结构电解质(BSSE),同时保持紧凑的内部溶剂化鞘和限制高温下的多硫化物穿梭。低温透射电镜结合x射线光电子能谱深度剖面揭示了固体电解质界面内层中丰富的无机成分。因此,具有BSSE的Li-S电池可以在60°C下保持90%的高容量保持稳定运行,并且在20-80°C的宽温度范围内也可以获得稳定的循环性能。我们的研究为研究Li-S电池及其以后的液体电解质化学提供了一个可靠的工具箱,为推进极端温度电解质的设计开辟了一条新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
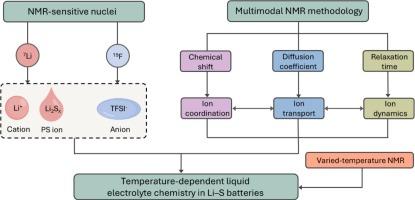
Modulating electrolyte solvation structure and ion dynamics for thermotolerant Li–S batteries
Traditional electrolytes impose tremendous limitation on the effective operation of lithium–sulfur (Li–S) batteries at elevated temperatures due to insufficient thermal stability and aggravated side reactions, wherein battery failure mechanism and electrolyte design principle remain elusive. Herein, we developed a varied-temperature multimodal nuclear magnetic resonance (VT-mNMR) methodology to elucidate temperature-dependent electrolyte solvation structure and ion dynamics, whereby a thermotolerant bistratal solvation structure electrolyte (BSSE) was formulated to concurrently maintain compact inner solvation sheath and restrict polysulfide shuttling at high temperatures. Cryogenic transmission electron microscopy combined with X-ray photoelectron spectroscopy depth profiling discloses rich inorganic components in the inner layer of solid electrolyte interface. Consequently, Li–S batteries with BSSE can sustain stable operation with a high capacity retention of 90 % at 60 °C, which also harvest a stable cycling performance under a wide temperature range within 20–80 °C. Our study provides a reliable toolbox for studying liquid electrolyte chemistry in Li–S batteries and beyond, which opens a new avenue for advancing extreme-temperature electrolyte design.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today
工程技术-材料科学:综合
CiteScore
36.30
自引率
1.20%
发文量
237
审稿时长
23 days
期刊介绍:
Materials Today is the leading journal in the Materials Today family, focusing on the latest and most impactful work in the materials science community. With a reputation for excellence in news and reviews, the journal has now expanded its coverage to include original research and aims to be at the forefront of the field.
We welcome comprehensive articles, short communications, and review articles from established leaders in the rapidly evolving fields of materials science and related disciplines. We strive to provide authors with rigorous peer review, fast publication, and maximum exposure for their work. While we only accept the most significant manuscripts, our speedy evaluation process ensures that there are no unnecessary publication delays.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


