SDMA(红铝玻璃体合化物):一种化学选择性和安全的还原反应溶液
IF 3.5
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
SDMA发现于1965年,已成为有机合成中的关键还原剂,以其选择性,稳定性和比传统氢化物(如锂铝氢化(LiAlH4, LAH)和二异丁基氢化铝(DIBAL-H))更高的安全性而闻名。它可以在学术和工业环境中实现有效的削减,包括选择性和部分削减。这篇综述探讨了它的各种应用,强调了它的优点,并提供了与它的使用相关的各种检查程序的详细见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
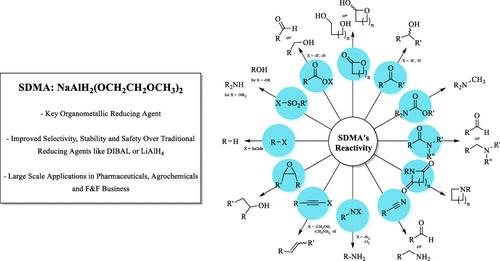
SDMA (Synhydrid, Red-Al, Vitride): A Chemoselective and Safe Solution for Reduction Reactions
Discovered in 1965, SDMA has become a key reducing agent in organic synthesis, known for its selectivity, stability, and improved safety over traditional hydrides such as lithium aluminum hydride (LiAlH4, LAH) and diisobutylaluminum hydride (DIBAL-H). It enables efficient reductions, including selective and partial reductions, in both academic and industrial settings. This review explores its diverse applications, highlights its advantages, and provides detailed insights into the various workup procedures associated with its use.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


