碘乙酰胺介导的协同溶剂界面调制使能无枝晶锌金属阳极
IF 4.3
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
锌离子电池(AZIBs)中锌金属阳极的可逆性和稳定性受到枝晶形成和寄生反应的限制。为了解决这些问题,碘乙酰胺(IAM)作为一种高效环保的电解质添加剂被引入,以实现卓越的长寿命azib。IAM的羰基和酰胺基协同重建Zn2+溶剂化壳,同时破坏氢键网络,从而减少水诱导的寄生反应。IAM在Zn表面的优先吸附有效地抑制了析氢反应(HER),并触发了含有SO32-、ZnS和zn3n2基化合物的坚固的固体电解质间相(SEI)层的形成。Zn3N2具有优异的离子电导率,促进了离子的快速传递动力学,而硫化复合材料有利于水合Zn2+离子的脱溶和疏水性的H2O排斥,协同降低了界面能垒,抑制了寄生副反应,从而实现了高度稳定和无枝晶的Zn沉积。添加IAM的Zn||对称电池在1 mA cm−2下的循环稳定性超过4500 h,在放电深度(DOD)为85.4 %时的循环稳定性为300 h。在10 a g−1下循环25000次后,Zn||PANI全电池的容量保持率高达85% %。本文章由计算机程序翻译,如有差异,请以英文原文为准。

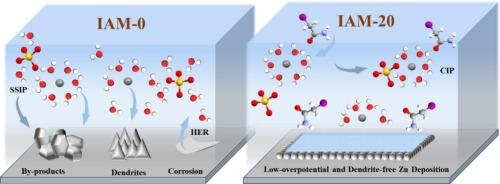
Dendrite-free Zn metal anodes enabled by iodoacetamide-mediated synergistic solvation-interface modulation
The reversibility and stability of zinc (Zn) metal anodes in aqueous zinc-ion batteries (AZIBs) are constrained by dendrite formation and parasitic reactions. To address these issues, iodoacetamide (IAM) is introduced as an efficacious and eco-friendly electrolyte additive to achieve superior long-life AZIBs. The carbonyl and amide groups of IAM coordinatively reconstruct the Zn2+ solvation shell while disrupting hydrogen-bond networks, thereby reducing water-induced parasitic reactions. The preferential adsorption of IAM on Zn surfaces effectively suppresses hydrogen evolution reactions (HER) and triggers the formation of a robust solid electrolyte interphase (SEI) layer containing SO32-, ZnS, and Zn3N2-based compounds. Zn3N2, with its exceptional ionic conductivity, promotes the rapid ion transport kinetics, while sulfurized composites facilitate the desolvation of hydrated Zn2+ ions and hydrophobicity for H2O repulsion, synergistically enabling reduced interfacial energy barriers, suppressed parasitic side reactions, and thereby achieving highly stable and dendrite-free Zn deposition. The Zn||Zn symmetric cell with the IAM additive demonstrates a remarkable cycling stability of over 4500 h at 1 mA cm−2 and an excellent cycle stability of 300 h at a depth of discharge (DOD) of 85.4 %. The Zn||PANI full cell maintains a high capacity retention of 85 % after 25,000 cycles at 10 A g−1.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


