叔丁醇促进4-硝基异恶唑类胺的亚硝化:5-氰基异恶唑的合成及其应用
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
已经开发了一种以前未知的5-氰基-4-硝基异恶唑的有效方法,使用亚硝基叔丁基(TBN)亚硝化4-硝基异恶唑基胺,然后是BF3或tfa介导的异常C = C键裂解反应。这种两步一锅工艺适用于广泛的底物,并在温和的反应条件下以中高产量提供所需的产品。在SNAr反应中,5-氰基-4-硝基异恶唑的反应活性的二元性已经被n -亲核试剂取代的氰基和噻吩取代的硝基所证明,并通过dft计算合理化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
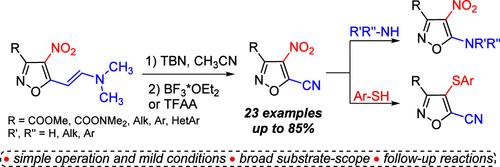
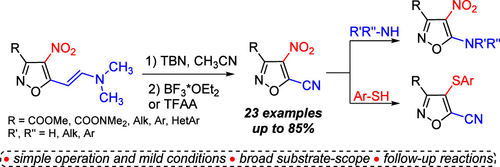
tert-BuONO-Promoted Nitrosation of 4-Nitroisoxazole-Based Enamines: Synthesis of 5-Cyanoisoxazoles and Their Application
An efficient approach to previously unknown 5-cyano-4-nitroisoxazoles has been developed using nitrosation of 4-nitroisoxazole-based enamines with tert-butyl nitrite (TBN) followed by a BF3- or TFAA-mediated unusual C═C bond cleavage reaction. This two-step one-pot process is applicable to a broad range of substrates and provides the desired products in mild reaction conditions in moderate to high yields. The dichotomy of reactivity in the SNAr reaction of the resulting 5-cyano-4-nitroisoxazoles has been demonstrated by the cyano-group substitution with N-nucleophiles and the nitro-group substitution with thiophenols and rationalized with DFT-calculations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


