多组学分析揭示维甲酸分子驱动真皮纤维化和皮肤再生修复
IF 20.4
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
摘要
皮肤纤维化是由成纤维细胞激活和过度的细胞外基质沉积驱动的。为了确定引起纤维化的成纤维细胞亚群,我们采用了一个已建立的小鼠博来霉素皮肤纤维化模型。我们通过多组学方法描述了真皮纤维化的纤维化和重塑阶段。使用无监督机器学习算法量化294个纤维特征,我们确定了纤维化和再生的精确时间点。单细胞转录组和表观基因组测序鉴定了一个表达cyp26b1的成纤维细胞亚群,负责皮肤纤维化。相同的成纤维细胞亚型被映射到Visium空间转录组数据中。我们进一步将纤维化亚型映射到蛋白质空间数据。为了确定成纤维细胞亚群的功能影响,移植递送分析显示了它们驱动皮肤纤维化的能力。最后,我们发现了一种Cyp26b1的小分子抑制剂(塔拉唑),可以预防和挽救皮肤纤维化。最后,我们建立了皮肤纤维化的纤维化和再生生物驱动的图谱。本文章由计算机程序翻译,如有差异,请以英文原文为准。
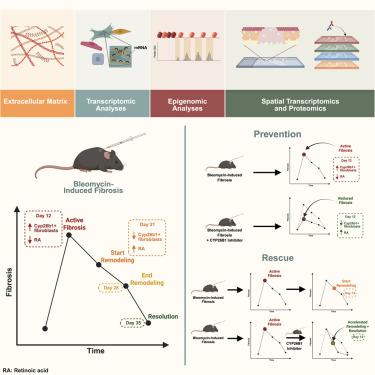
Multi-omic analysis reveals retinoic acid molecular drivers for dermal fibrosis and regenerative repair in the skin
Skin fibrosis is driven by fibroblast activation and excessive extracellular matrix deposition. To ascertain the fibroblast subpopulation(s) responsible for instigating fibrosis, we employed an established murine bleomycin skin fibrosis model. We characterized both the fibrotic and remodeling phases of dermal fibrosis through a multi-omic approach. Using an unsupervised machine learning algorithm that quantifies 294 fiber features, we identified precise time points of fibrosis and regeneration. Single-cell transcriptomic and epigenomic sequencing then identified a Cyp26b1-expressing fibroblast subpopulation responsible for dermal fibrosis. The same fibroblast subtype was mapped to Visium spatial transcriptomic data. We further mapped the fibrotic subtypes to protein spatial data. To ascertain the functional impact of the fibroblast subpopulations, transplant delivery analysis showed their ability to drive skin fibrosis. Lastly, we identified a small molecular inhibitor of Cyp26b1 (talarozole) that prevents and rescues dermal fibrosis. Conclusively, we establish an atlas of the fibrotic and regenerative biological drivers of skin fibrosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


