对极端高温反应的分子机制:细胞骨架动力学和免疫激活适应的见解
IF 3.7
2区 农林科学
Q1 FISHERIES
引用次数: 0
摘要
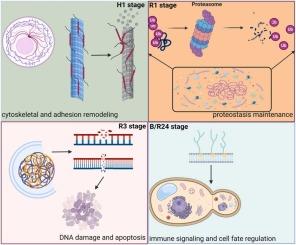
海洋变暖严重威胁着冷水双壳类动物的生存和生态稳定。然而,极端高温胁迫下分子损伤和恢复的时间动力学仍然知之甚少。在这项研究中,我们检测了极端高温处理(30°C, H1)后1 h以及恢复期(25°C,可选:R1, R3, R24) 1, 3和24 h时叶氏Patinopecten yessoensis鳃的转录组学和生理反应。结果表明,极端高温胁迫导致叶青广泛的、延迟的转录中断,突出了其分子响应和恢复动力学。在恢复阶段,R3阶段作为一个关键的过渡窗口出现,在此期间细胞稳态开始恢复,关键的修复途径被重新激活。此外,WGCNA揭示的顺序恢复轨迹表明,恢复始于早期的细胞骨架不稳定和钙依赖信号传导(H1, Myellow模块),通过蛋白质质量控制和内质网稳态(R1, Mbrown模块)进行,最终在细胞死亡调节、免疫信号传导和组织重塑(R3, Mblue模块)中结束。微管系统被确定为热破坏的主要目标,在应力后立即表现出下调和结构解体,并在R3阶段恢复。这些发现共同表明,叶杉采用多层适应策略来抵御和从温度诱导的损伤中恢复,细胞骨架完整性、基因组维持和程序性细胞死亡之间的时间协调受到严格调节。该研究加深了我们对冷水贝类高温响应机制的认识,对冷水贝类养殖的生态风险评估和选择育种具有重要意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Molecular mechanisms of Patinopecten yessoensis responding to extreme high temperature: insights into cytoskeletal dynamics and immune activation adaptation
Ocean warming critically threatens the survival and ecological stability of cold-water bivalves. Yet, the temporal dynamics of molecular damage and recovery following extreme high temperature stress remain poorly understood. In this study, we examined the transcriptomic and physiological responses of Patinopecten yessoensis in gills at 1 h after extreme high temperature treatment (30 °C, H1) and at 1, 3 and 24 h during the recovery period ( 25 °C, alternatives: R1, R3, R24). The results revealed that extreme high-temperature stress caused widespread, delayed transcriptional disruption in P. yessoensis, highlighting its molecular response and recovery dynamics. During the recovery stages, and the R3 stage emerged as a critical transition window during which cellular homeostasis began to be restored, and key repair pathways were reactivated. Furthermore, a sequential recovery trajectory revealed by WGCNA indicated that the recovery began with early cytoskeletal destabilization and calcium-dependent signaling (H1, Myellow module), progressed through protein quality control and endoplasmic reticulum homeostasis (R1, Mbrown module), and culminated in cell death regulation, immune signaling, and tissue remodeling (R3, Mblue module). The microtubule system was identified as a primary target of thermal disruption, showing downregulation and structural disassembly immediately after stress and recovering in R3 stage. These findings collectively suggested that P. yessoensis employs a multi-tiered adaptive strategy to withstand and recover from temperature-induced injury, with tightly regulated temporal coordination between cytoskeletal integrity, genomic maintenance, and programmed cell death. This study enhances our understanding in high temperature response mechanism of cold-water bivalves, and have significant implications for ecological risk assessment and selective breeding in cold-water shellfish aquaculture.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Aquaculture Reports
Agricultural and Biological Sciences-Animal Science and Zoology
CiteScore
5.90
自引率
8.10%
发文量
469
审稿时长
77 days
期刊介绍:
Aquaculture Reports will publish original research papers and reviews documenting outstanding science with a regional context and focus, answering the need for high quality information on novel species, systems and regions in emerging areas of aquaculture research and development, such as integrated multi-trophic aquaculture, urban aquaculture, ornamental, unfed aquaculture, offshore aquaculture and others. Papers having industry research as priority and encompassing product development research or current industry practice are encouraged.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


