木犀叶茎叶化学成分的研究
IF 1.4
4区 生物学
Q4 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
我们的调查强调了天然产物在药物发现中的持续重要性。我们报道了一种新的咔唑生物碱(1)和18个已知的化合物(2 - 19)的分离,以确定潜在的抗肿瘤药物。这些化合物的结构通过核磁共振、质谱、电子圆二色性(ECD)和核磁共振计算等多种光谱分析得以阐明。分离的化合物随后通过亲和力筛选评估其对血管内皮生长因子受体-2 (VEGFR-2)的亲和力。采用混合lamarkian遗传算法(LGA)进行分子对接模拟,选择结合能在−6.9至−10.1 kcal/mol之间的构象,结果显示化合物1、6、8、10−14、16和19与VEGFR-2蛋白具有显著的亲和力。此外,化合物1、6、8、13和19与VEGFR-2具有多个结合位点,而化合物10的结合能最低(−10.1 kcal/mol)。这些发现为进一步研究这些化合物的抗肿瘤机制提供了坚实的基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
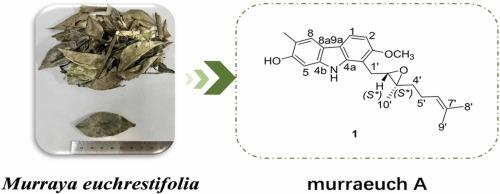
Studies on the chemical constituents of the stems and leaves of Murraya euchrestifolia
The ongoing importance of natural products in drug discovery is highlighted by our investigation. We report the isolation of a novel carbazole alkaloid (1) and 18 known compounds (2−19) from Murraya euchrestifolia to identify potential anti-tumor agents. The structures of these compounds were elucidated through various spectroscopic analysis including NMR, MS, electronic circular dichroism (ECD), and NMR calculations. The isolated compounds were subsequently evaluated for their affinity towards the vascular endothelial growth factor receptor-2 (VEGFR-2) through affinity screening. Molecular docking simulations, employing a hybrid Lamarckian Genetic Algorithm (LGA) to select conformations with binding energies ranging from −6.9 to −10.1 kcal/mol, revealed that compounds 1, 6, 8, 10−14, 16, and 19 exhibited significant affinity for the VEGFR-2 protein. Furthermore, compounds 1, 6, 8, 13, and 19 demonstrated multiple binding sites with VEGFR-2, while compound 10 displayed the lowest binding energy (−10.1 kcal/mol). These findings provided a solid foundation for further investigation into the anti-tumor mechanisms of these compounds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytochemistry Letters
生物-生化与分子生物学
CiteScore
3.00
自引率
11.80%
发文量
190
审稿时长
34 days
期刊介绍:
Phytochemistry Letters invites rapid communications on all aspects of natural product research including:
• Structural elucidation of natural products
• Analytical evaluation of herbal medicines
• Clinical efficacy, safety and pharmacovigilance of herbal medicines
• Natural product biosynthesis
• Natural product synthesis and chemical modification
• Natural product metabolism
• Chemical ecology
• Biotechnology
• Bioassay-guided isolation
• Pharmacognosy
• Pharmacology of natural products
• Metabolomics
• Ethnobotany and traditional usage
• Genetics of natural products
Manuscripts that detail the isolation of just one new compound are not substantial enough to be sent out of review and are out of scope. Furthermore, where pharmacology has been performed on one new compound to increase the amount of novel data, the pharmacology must be substantial and/or related to the medicinal use of the producing organism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


