微塑料在可持续制药生产中的应用
IF 5.8
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
微塑料(MPs),小于5毫米的塑料颗粒,是环境和制药领域关注的新兴污染物。在药品生产中,MPs通常源于塑料设备、包装材料和渗滤液的分解,并可在生产、处理或储存过程中进入药品。本文从可持续制造和绿色化学原理的角度探讨了这些污染途径。MPs存在于制剂中,特别是液体和半固体剂型中,会通过影响活性药物成分(api)的稳定性、溶解度和生物利用度而损害药物功效。MPs还可能是有毒物质的载体,如重金属和干扰内分泌的化学物质,增加了对人类健康和环境的潜在风险。结合生物循环经济概念,该综述评估了先进的检测技术,包括FTIR,拉曼光谱和SEM,以及可持续的样品制备方法。它还强调了符合良好生产规范(GMP)的污染控制措施,例如使用非塑料加工工具,无尘室改进和环保包装,所有这些都旨在减少MPs污染并支持循环材料的使用。这种多学科方法强调需要符合联合国可持续发展目标的可持续的、基于系统的解决方案。为了推进这些工作,未来的研究应侧重于标准化检测方法,研究MPs对药物安全的长期影响,以及开发实时监测工具。此外,明确的监管准则和针对药品中微塑料的风险评估模型对于指导有效的行业实践和政策决定至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
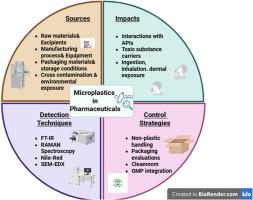
Microplastics in sustainable pharmaceutical manufacturing
Microplastics (MPs), plastic particles smaller than 5 mm, are emerging contaminants of concern in both environmental and pharmaceutical fields. Within pharmaceutical manufacturing, MPs typically originate from the breakdown of plastic-based equipment, packaging materials, and leachates, and can enter drug products during production, handling, or storage. This review explores these contamination pathways through the perspective of sustainable manufacturing and green chemistry principles. The presence of MPs in formulations particularly liquid and semi-solid dosage forms can compromise drug efficacy by affecting the stability, solubility, and bioavailability of active pharmaceutical ingredients (APIs). MPs may also serve as carriers of toxic substances, such as heavy metals and endocrine-disrupting chemicals, increasing potential risks to human health and the environment. Incorporating bio-circular economy concepts, the review evaluates advanced detection techniques including FTIR, Raman spectroscopy, and SEM, along with sustainable sample preparation methods. It also highlights contamination control measures aligned with Good Manufacturing Practices (GMP), such as the use of non-plastic processing tools, cleanroom improvements, and eco-friendly packaging all aimed at reducing MPs contamination and supporting circular material use. This multidisciplinary approach emphasizes the need for sustainable, system-based solutions that align with the United Nations Sustainable Development Goals. To advance these efforts, future research should focus on standardizing detection methods, studying long-term impacts of MPs on drug safety, and developing real-time monitoring tools. Additionally, clear regulatory guidelines and risk assessment models specific to microplastics in pharmaceuticals are essential for guiding effective industry practices and policy decisions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Sustainable Chemistry and Pharmacy
Environmental Science-Pollution
CiteScore
8.20
自引率
6.70%
发文量
274
审稿时长
37 days
期刊介绍:
Sustainable Chemistry and Pharmacy publishes research that is related to chemistry, pharmacy and sustainability science in a forward oriented manner. It provides a unique forum for the publication of innovative research on the intersection and overlap of chemistry and pharmacy on the one hand and sustainability on the other hand. This includes contributions related to increasing sustainability of chemistry and pharmaceutical science and industries itself as well as their products in relation to the contribution of these to sustainability itself. As an interdisciplinary and transdisciplinary journal it addresses all sustainability related issues along the life cycle of chemical and pharmaceutical products form resource related topics until the end of life of products. This includes not only natural science based approaches and issues but also from humanities, social science and economics as far as they are dealing with sustainability related to chemistry and pharmacy. Sustainable Chemistry and Pharmacy aims at bridging between disciplines as well as developing and developed countries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


