NiO催化剂诱导苝预成核的分子动力学研究
IF 2.4
3区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
有机纳米晶体(ONCs)由于其独特的性质在光电子学中至关重要,但其形成的早期阶段,特别是其在预成核中的催化作用尚不清楚。本研究采用分子动力学模拟方法探讨了NiO纳米颗粒(NPs)对苝基ONCs(特别是PERLEN08和RELVUC)预成核的影响。结果表明,NiO NPs显著改变了团簇形成速率和稳定性。与未催化的体系相比,催化剂的存在引入了关键的动力学、热力学和结构差异。由于分子结构和氢键的吸附屏障不同,PERLEN08在NiO存在下比RELVUC形成簇的速度更快。NiO还增强了这两个系统的集群稳定性。结构分析表明,主要是非晶态的预成核团簇,支持非经典成核机制。通过提供催化剂选择性的原子尺度机制,这些发现有助于解释底物依赖成核的实验结果,并为合理设计控制ONC合成的催化剂提供基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
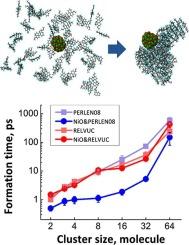
A molecular dynamics study of NiO catalyst-induced perylene prenucleation
Organic nanocrystals (ONCs) are pivotal in optoelectronics due to their unique properties, yet the early stages of their formation, particularly their catalytic role in prenucleation, are unclear. This study uses molecular dynamics simulations to explore the effects of NiO nanoparticles (NPs) on the prenucleation of perylene-based ONCs, specifically PERLEN08 and RELVUC. Results show that NiO NPs significantly alter cluster formation rates and stability. The catalyst's presence introduces key kinetic, thermodynamic, and structural differences compared to the uncatalyzed system. PERLEN08 forms clusters faster than RELVUC in the presence of NiO, attributed to differing adsorption barriers from molecular structure and hydrogen bonding. NiO also enhances cluster stability for both systems. Structural analysis indicates predominantly amorphous pre-nucleation clusters, supporting non-classical nucleation mechanisms. By providing an atomic-scale mechanism for catalyst selectivity, these findings help interpret experimental results on substrate-dependent nucleation and provide a basis for the rational design of catalysts to control ONC synthesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Physics
化学-物理:原子、分子和化学物理
CiteScore
4.60
自引率
4.30%
发文量
278
审稿时长
39 days
期刊介绍:
Chemical Physics publishes experimental and theoretical papers on all aspects of chemical physics. In this journal, experiments are related to theory, and in turn theoretical papers are related to present or future experiments. Subjects covered include: spectroscopy and molecular structure, interacting systems, relaxation phenomena, biological systems, materials, fundamental problems in molecular reactivity, molecular quantum theory and statistical mechanics. Computational chemistry studies of routine character are not appropriate for this journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


