长保质期异体脂肪来源的间充质干细胞系来源的血小板样细胞治疗难治性足溃疡的安全性和有效性:一项转化性临床前和1/2a期研究
IF 3.5
3区 环境科学与生态学
Q3 CELL & TISSUE ENGINEERING
引用次数: 0
摘要
难治性足部溃疡的特点是愈合时间长,复发频繁,对现有的治疗方法(如血运重建术和压迫术)提出了挑战,这些治疗方法通常不能防止截肢。我们开发了脂肪来源的间充质干细胞系衍生的血小板样细胞(ascl - plc),用于治疗难治性溃疡,这种细胞持久且容易获得。方法一项临床前研究和1/2a期首次人体临床研究评估了一种新型异体ASCL-PLC治疗难治性足部溃疡的安全性和有效性。在临床前阶段,ascl - plc冷冻保存9个月,然后解冻,以测量氯化钙刺激后细胞因子的释放。采用糖尿病小鼠创面模型,比较ASCL-PLC治疗与对照治疗的疗效。临床试验包括在第0、14和28天将ascl - plc (1.5 × 108细胞/cm2)局部应用于4例患者(2例缺血性,2例静脉)的溃疡,并根据不良事件、溃疡大小缩小、上皮化时间和疼痛评分变化监测安全性和有效性。结果ascl - plc在长期冷冻保存后仍保持相当高的细胞因子释放活性。在糖尿病小鼠皮肤缺损模型中,与对照组相比,ascl - plc治疗显著增强了伤口愈合,显示出强大的伤口愈合能力。在临床试验中,所有患者均表现出明显的溃疡缩小,无严重不良事件;3例患者在6个月内实现了上皮化,疼痛和生活质量得到改善,且对局部血流没有负面影响。结论sascl - plc是一种安全且有前景的治疗顽固性溃疡的选择,可以潜在地降低截肢率,改善患者预后,并最大限度地减少慢性伤口造成的社会经济影响。它们的长保质期和低温保存稳定性使伤口管理实用和方便。此外,他们的疗效与简单的局部应用建议微创策略适合门诊护理。建议进一步进行大规模试验。本研究已在日本临床试验注册中心注册(jRCT ID: jRCTa030200053;2020年6月18日,https://jrct.mhlw.go.jp/en-latest-detail/jRCTa030200053)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
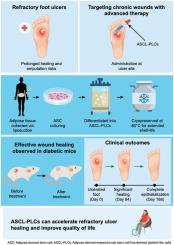
Safety and efficacy of long-shelf-life allogeneic adipose-derived mesenchymal stem cell line-derived platelet-like cells for refractory foot ulcers: A translational preclinical and phase 1/2a study
Introduction
Refractory foot ulcers, characterized by prolonged healing and frequent recurrences, challenge existing therapies such as revascularization and compression, which typically fail to prevent amputation. We developed adipose-derived mesenchymal stem cell line-derived platelet-like cells (ASCL-PLCs) for refractory ulcers that are long-lasting and readily available.
Methods
A translational preclinical investigation and phase 1/2a first-in-human clinical study assessed the safety and efficacy of a novel allogeneic ASCL-PLC treatment for refractory foot ulcers. In the pre-clinical phase, ASCL-PLCs were cryopreserved for 9 months and then thawed to measure cytokine release after calcium chloride stimulation. The therapeutic efficacy was evaluated in a diabetic mouse wound model, comparing ASCL-PLC treatment with vehicle control. The clinical trial involved topically applying ASCL-PLCs (1.5 × 108 cells/cm2) to the ulcers of four patients (two ischemic, two venous) on days 0, 14, and 28, with safety and efficacy monitored based on adverse events, ulcer size reduction, epithelialization time, and pain score changes.
Results
ASCL-PLCs maintained considerable cytokine-releasing activity even after long-term cryopreservation. In the diabetic mouse skin defect model, treatment with ASCL-PLCs substantially enhanced wound closure compared with the controls, demonstrating potent wound-healing capabilities. In the clinical trial, all patients exhibited notable ulcer size reduction without serious adverse events; three achieved epithelialization within 6 months, along with improvements in pain and quality of life without a negative impact on local blood flow.
Conclusions
ASCL-PLCs are safe and a promising therapeutic option for refractory ulcers and can potentially reduce amputation rates, improve patient outcomes, and minimize the socioeconomic impact due to chronic wounds. Their long shelf life and cryopreservation stability make wound management practical and accessible. Moreover, their efficacy with simple topical application suggests a minimally invasive strategy suitable for outpatient care. Further large-scale trials are recommended.
This study was registered with the Japan Registry of Clinical Trials (jRCT ID: jRCTa030200053; on June 18, 2020, https://jrct.mhlw.go.jp/en-latest-detail/jRCTa030200053).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Regenerative Therapy
Engineering-Biomedical Engineering
CiteScore
6.00
自引率
2.30%
发文量
106
审稿时长
49 days
期刊介绍:
Regenerative Therapy is the official peer-reviewed online journal of the Japanese Society for Regenerative Medicine.
Regenerative Therapy is a multidisciplinary journal that publishes original articles and reviews of basic research, clinical translation, industrial development, and regulatory issues focusing on stem cell biology, tissue engineering, and regenerative medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


